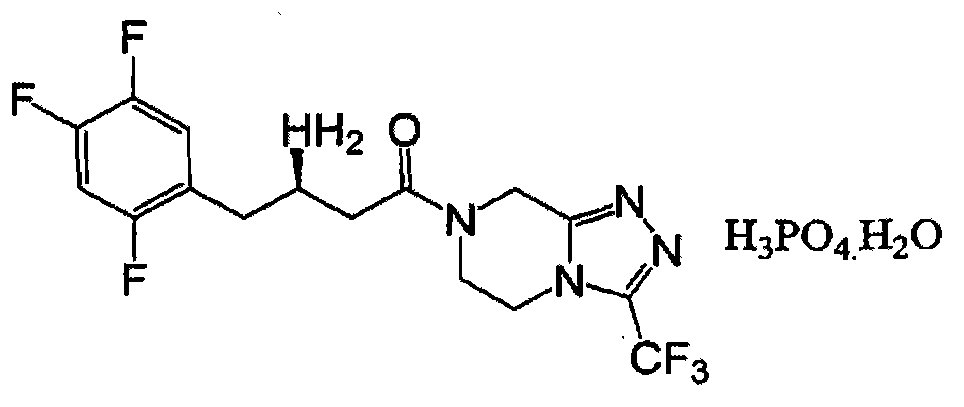
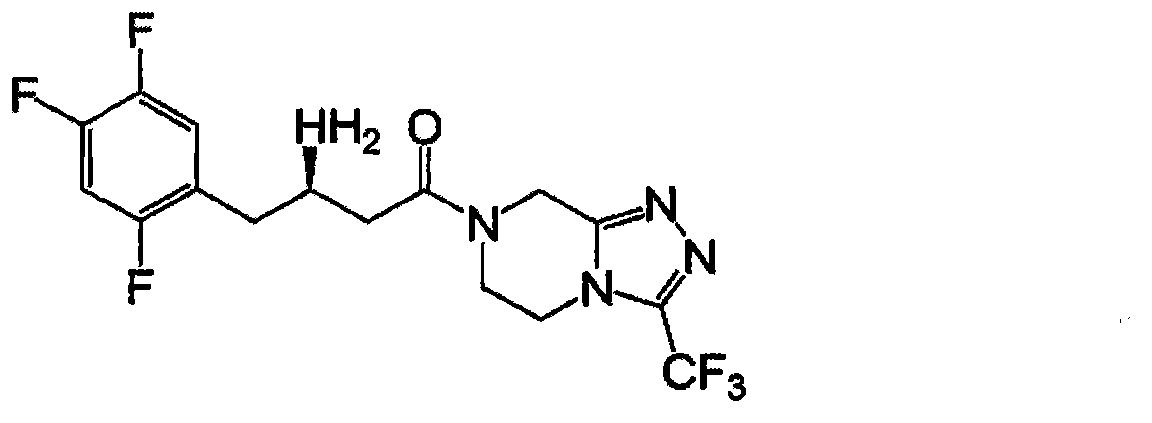
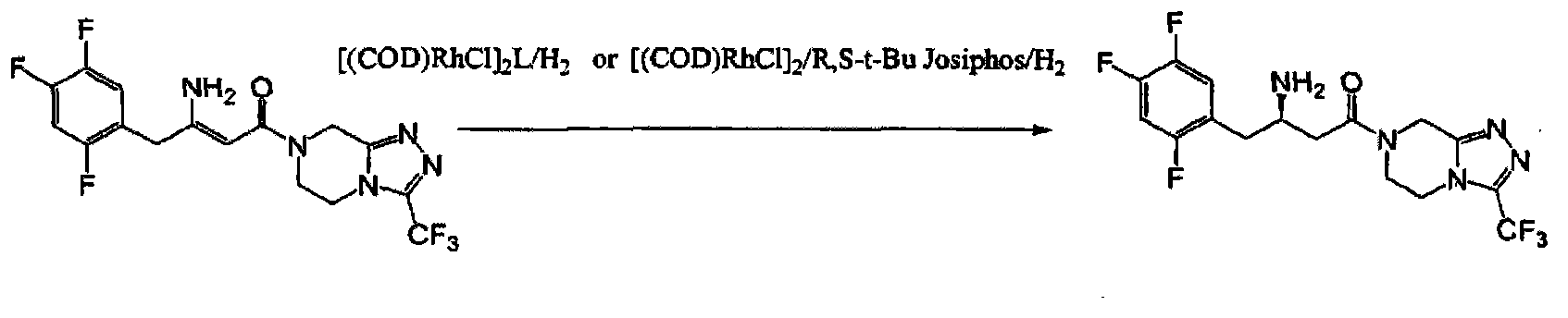
Industrial production method of sitagliptin
A technique for sitagliptin and a production method, which is applied in the field of industrial production of sitagliptin, can solve the problems of low chiral purity of the product, certain difficulty, complex process, etc., and achieve high yield, cost reduction, and high chirality The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] (1) Preparation of chiral catalyst: under nitrogen protection, add (1,5 cyclooctadiene) ruthenium dichloride 165.3mg (0.59mmol) and S-(-)-1,1 to a 500ml reaction flask, 367.4 mg (0.59 mmol) of -binaphthyl-2,2,-diphenylphosphine, 200 ml of deoxygenated methanol, and the mixture was stirred at room temperature for one hour for later use.
[0021] (2) Preparation of sitagliptin free base: add 7-[3-amino-1-oxygen-4-(2,4,5-trifluorophenyl)-2-butenyl to 4l autoclave ]-5,6,7,8-tetrahydro-3-(1-trifluoromethyl)-1,2,4-triazol[4,3,α]pyrazine 118g (0.29mol) and deoxymethanol l Replace with nitrogen 3 times under stirring, add the chiral catalyst solution prepared in (1), replace with nitrogen 3 times under stirring, close all valves, slowly raise the temperature to 50°C, open the hydrogen valve, and slowly introduce hydrogen When the hydrogen pressure rises to 150 psi, keep the pressure and keep the reaction for 12-36 hours. Take a sample in the middle and analyze it by HPLC until...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com