Nucleoside compounds with HIV-1/HBV viral replication inhibition activity, preparation methods thereof, and antiviral applications thereof
A compound, cyclic nucleoside technology, applied in the field of nucleoside compounds, can solve the problems of poor membrane permeability, sufficient concentration of infected parts, no antiviral activity, etc., and achieve good fat solubility, low toxicity, and high activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
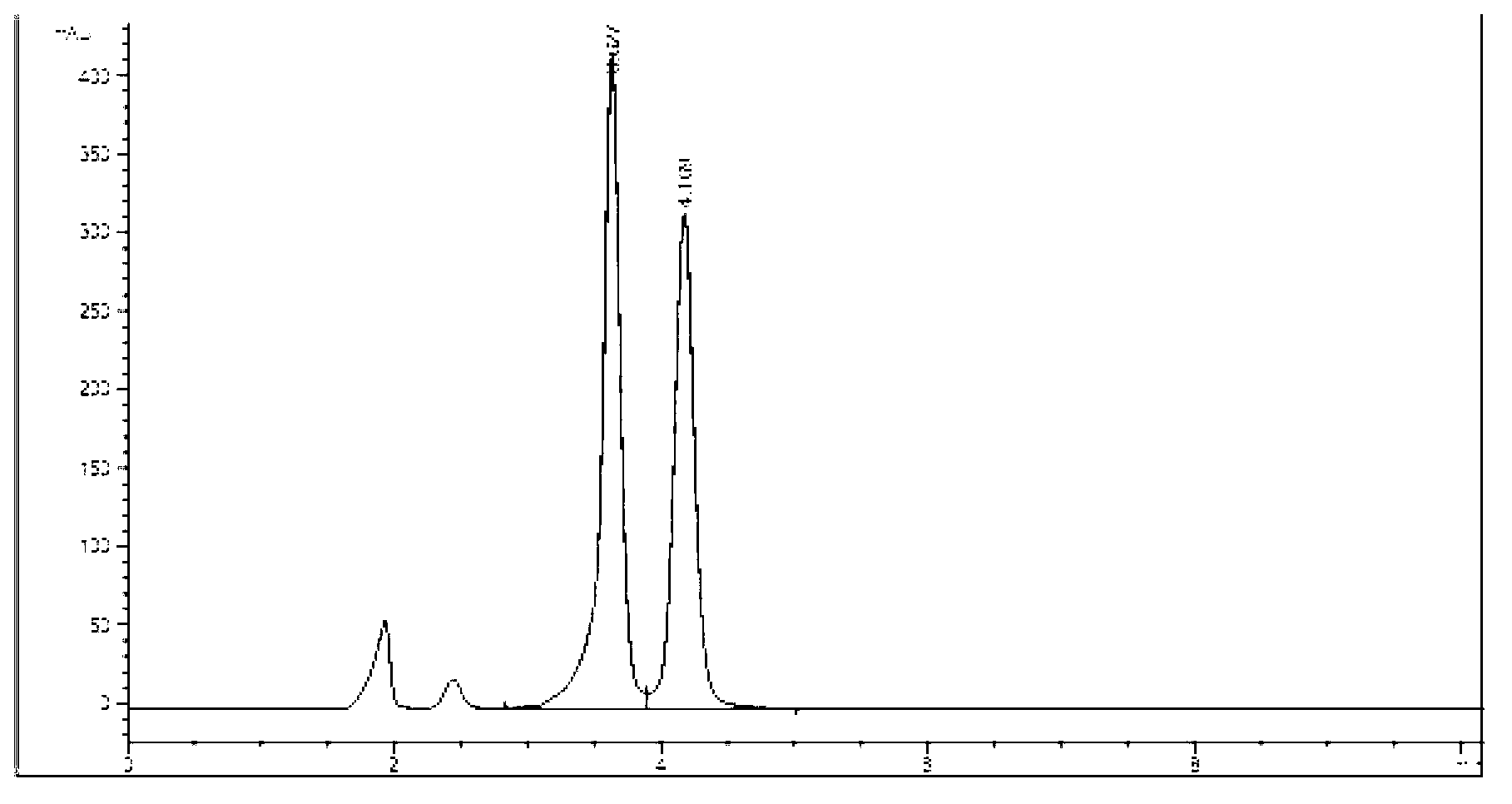
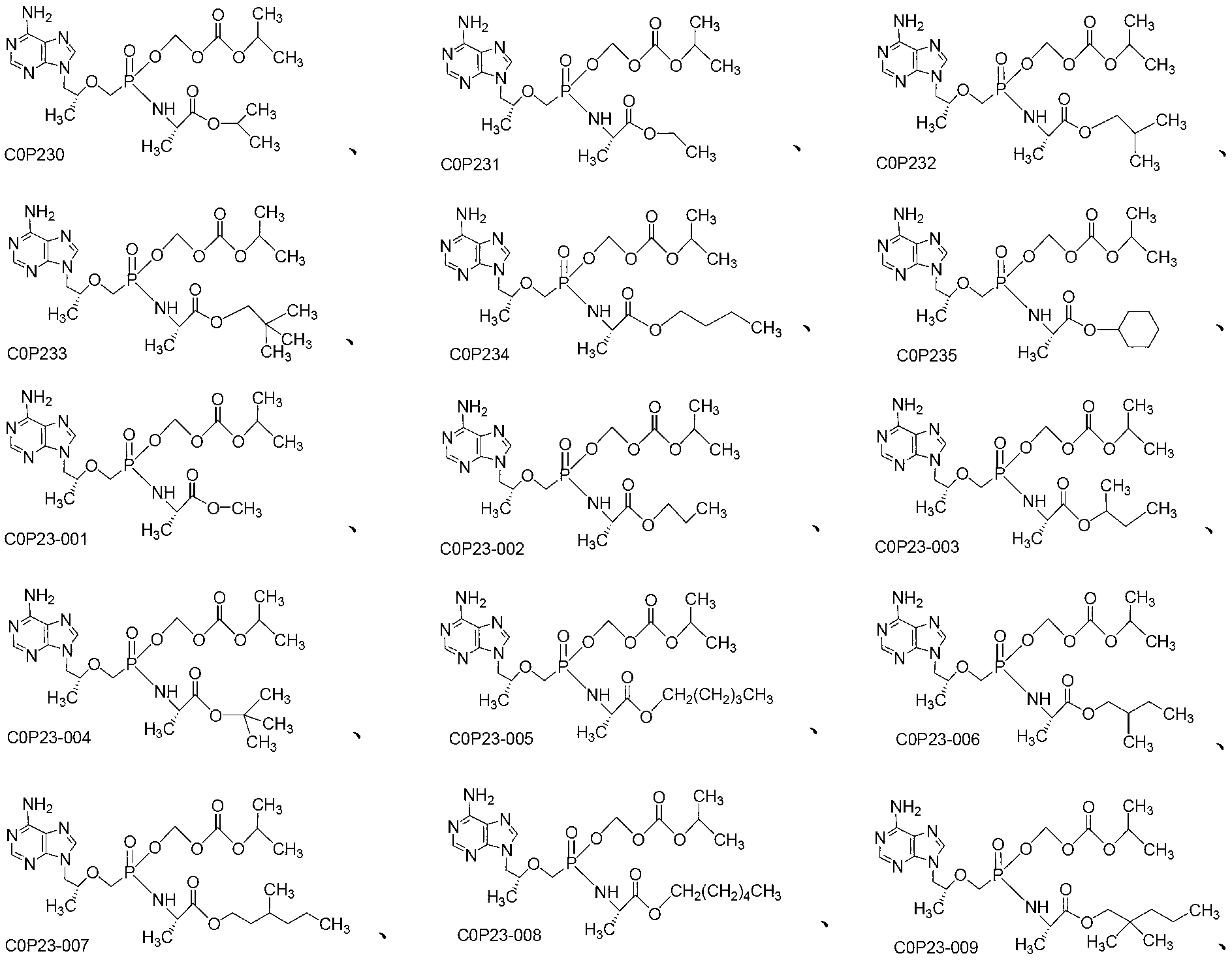
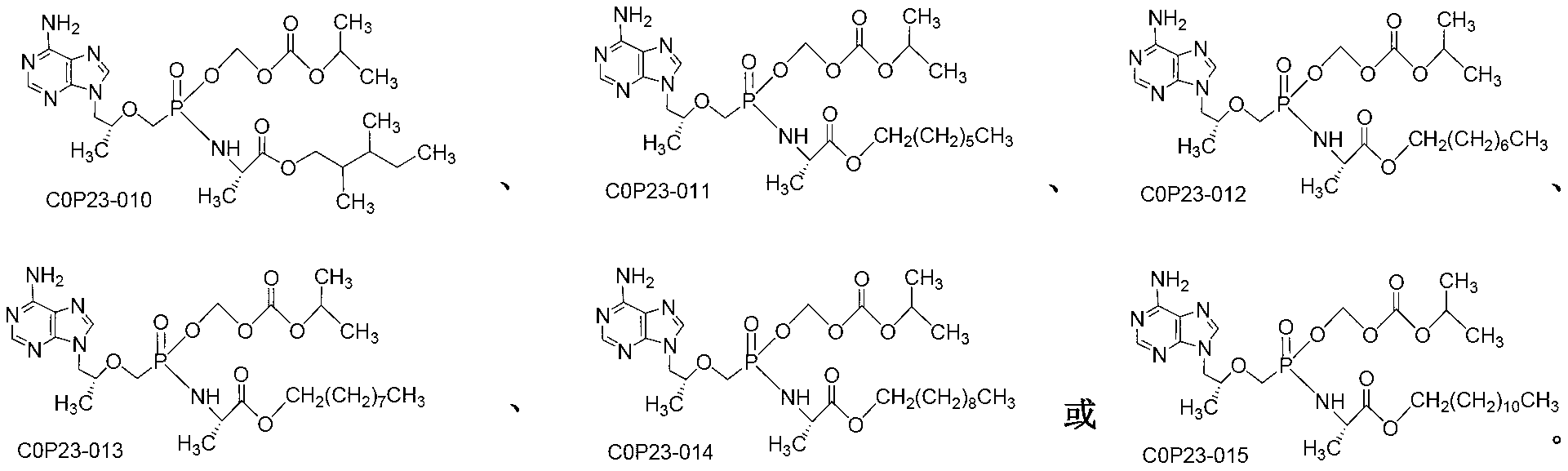
[0050] Embodiment 1: (R)-9-[2-[[isopropoxycarbonyloxymethyl[(S)-1-(ethoxycarbonyl)] ethyl] phosphoramidate methoxy] propyl] adenine (COP231 ) preparation
[0051]
[0052] In a 50ml round bottom flask, add (R)-9-[2-(phosphomethoxy)propyl]adenine (tenofovir, PMPA) (1.3g, 4.5mmol), triethylamine (7ml , 5.08g, 50.2mmol), chloromethyl isopropyl carbonate (0.265ml, 0.305g, 2mmol) and N-methyl-2-pyrrolidone (14ml), stirred at 60°C for 15h, evaporated the solvent, and used Ethyl acetate: ethanol=10:1 silica gel column chromatography, obtain intermediate compound: (R)-9-{2-[(isopropoxycarbonyloxymethyl) phosphomethoxy] propyl group} adenine (C0P02) (0.68g, 1.68mmol) yield 37.3%.
[0053] In a 50ml round bottom flask, add COP02 (8.47g, 21mmol), L-alanine ethyl ester hydrochloride (6.6g, 43mmol), 2,2'-dithiodipyridine (9.03g, 41mmol), Triethylamine (11.04g, 15.2ml, 109mmol), triphenylphosphine (11.2g, 43mmol) and pyridine (25ml), after closed stirring at 65°C for 16h, evaporate th...
Embodiment 2
[0054] Embodiment 2: (R)-9-[2-[[isopropoxycarbonyloxymethyl [(S)-1-(isobutoxycarbonyl)] ethyl] phosphoramidate methoxy] propyl] adenine ( Preparation of COP232)
[0055]
[0056] Synthesized in a similar manner to Example 1: (R)-9-[2-[[isopropoxycarbonyloxymethyl[(S)-1-(isobutoxycarbonyl)]ethyl]phosphoramidate methoxy]propane Base] adenine (COP232). 1 H NMR (400MHz, CDCl 3 )δ,(ppm):0.87-0.95(6H,m,2×CH 3 ), 1.17-1.45 (12H, m, 4×CH 3 ),1.84-2.00(1H,m,CH),3.60-3.71(1H,m,OCH),3.83-4.00(4H,m,COOCH,COOCH 2 and NCH), 4.08-4.18 (2H, m, OCH 2 P),4.25-4.46(2H,m,NCH 2 ),4.87-4.98(1H,m,NH),5.57-5.73(2H,m,OCH 2 O),6.60(2H,s,NH 2 ),7.96-8.03(1H,d,H on the purine ring),8.29-8.36(1H,d,H on the purine ring).ESI-MS:[M+H] + 531.3, [M+Na] + 553.2
Embodiment 3
[0057] Embodiment 3: (R)-9-[2-[[isopropoxycarbonyloxymethyl[(S)-1-(neopentyloxycarbonyl)] ethyl] phosphoramidate methoxy] propyl] adenine ( Preparation of COP233)
[0058]
[0059] Synthesized in a similar manner to Example 1: (R)-9-[2-[[isopropoxycarbonyloxymethyl[(S)-1-(neopentyloxycarbonyl)]ethyl]phosphoramidate methoxy]propane Base] adenine (COP233). 1 H NMR (400MHz, CDCl 3 )δ,(ppm):0.94(9H,s,3×CH 3 ),1.17-1.22(3H,d,CH 3 ), 1.26-1.32 (6H, m, 2×CH 3 ),1.40-1.46(3H,d,CH 3 ),3.60-3.87(3H,m,2×COOCH and NCH),3.88-3.95(2H,m,COOCH 2 ),4.08-4.39(4H,m,OCH 2 Pand NCH 2 ),4.86-4.97(1H,m,NH),5.57-5.73(2H,m,OCH 2 O),6.46(2H,s,NH 2 ),7.99(1H,s,H on the purine ring),8.33(1H,s,H on the purine ring).ESI-MS:[M+H] + 545.4, [M+Na] + 567.3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com