Evans blue complex as well as preparation method and application thereof
An Evans blue and complex technology, applied in the field of radioactive complexes, can solve the problems of susceptible virus imaging effect, susceptible to other factors, complicated preparation method, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
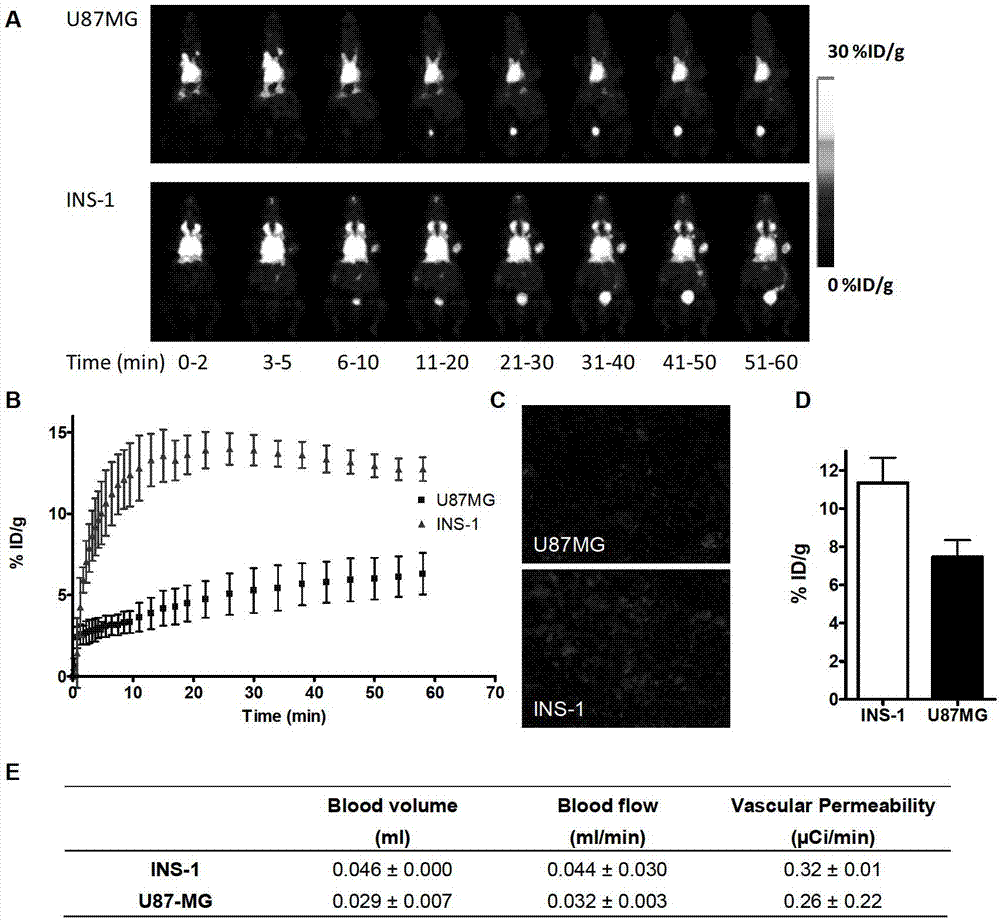
Image
Examples
Embodiment 1
[0071] 1. Preparation of Evans Blue Derivative Ligand (Compound 6)
[0072] 1) Synthesis of Compound 3:
[0073] Mix and dissolve 20.0 mg of compound 1 (ortho-dimethylbenzidine, 94 μmol) and 20.0 mg of compound 2 (NOTA-3HCl, 48 μmol) in 1 mL of DMSO, then add 3.6 μL of diethyl cyanophosphate (24 μmol) and 25 μL Diisopropylethylamine (DIPEA) was added to the above mixture, and after stirring at room temperature for 40 min, 3.6 μL of diethyl cyanophosphate (24 μmol) was added again, and the reaction mixture was kept stirring overnight at room temperature. The product (compound 3) was separated and purified by semi-preparative HPLC, and the fraction with a retention time of 10.0 min was collected and freeze-dried to obtain about 12.2 mg of pure compound 3 with a yield of 26.4%. The intermediate product was identified by liquid chromatography-mass spectrometry (LC-MS): [MH] + =498.2462, the calculated value (m / z) is 497.2638 (C 26 h 35 N 5 o 5 ).
[0074] 2) Synthesis of Co...
Embodiment 2
[0077] 1. radioactivity 18 Preparation of F-marked freeze-dried kit (take the preparation of 100 tubes as an example)
[0078] Weigh 8 mg of compound 6 and dissolve in 10 mL of 0.5 mol / L acetic acid-sodium acetate buffer solution (pH4), then add 0.08 mg of aluminum chloride (AlCl 3 ) was dissolved in 10mL of 0.5mol / L acetic acid-sodium acetate buffer solution (pH4), and the two were evenly mixed. After sterile filtration, it is divided into 100 control antibiotic vials, then placed in a freeze dryer for 24 hours, and then sealed with a stopper to obtain a freeze-dried medicine box. According to the output of the kit and the requirements for the content of the components in each kit, the amount of compound 6 and aluminum chloride can be adjusted so that their weight ratio falls within the range of (20-100):1.
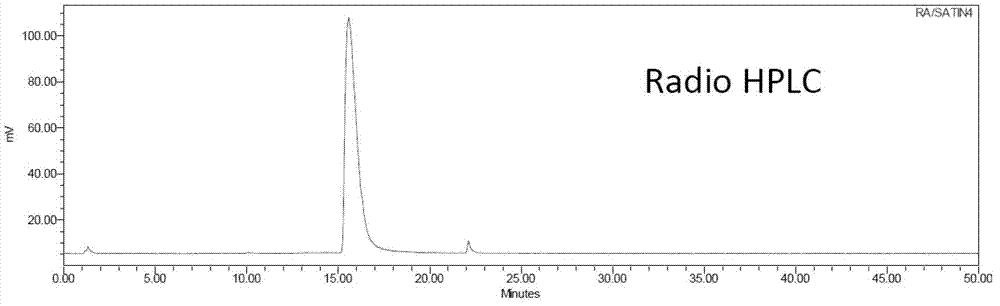
[0079] 2. 18 Preparation of F-labeled Evans blue complex (compound 7):
[0080] 1) Wet method: In a 1mL plastic tube, add 3μL of 2mM AlCl 3 Acetic acid-acetate buf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com