Method for preparing type b Haemophilus influenzae and polysaccharide conjugate vaccine
A technology of Haemophilus influenzae and conjugated vaccines, which is applied in the field of preparation of Haemophilus influenzae type b polysaccharide conjugated vaccines, which can solve the problems affecting the stability of the production process, the stability of finished vaccines between batches, the quality consistency of finished vaccines, the combination of high molecular weight polysaccharides and proteins Low product yield, uneven molecular size distribution, etc., to achieve the effect of saving raw materials, reducing production costs, and reducing differences between batches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A method for preparing Haemophilus influenzae type b polysaccharide conjugate vaccine, comprising the following steps:
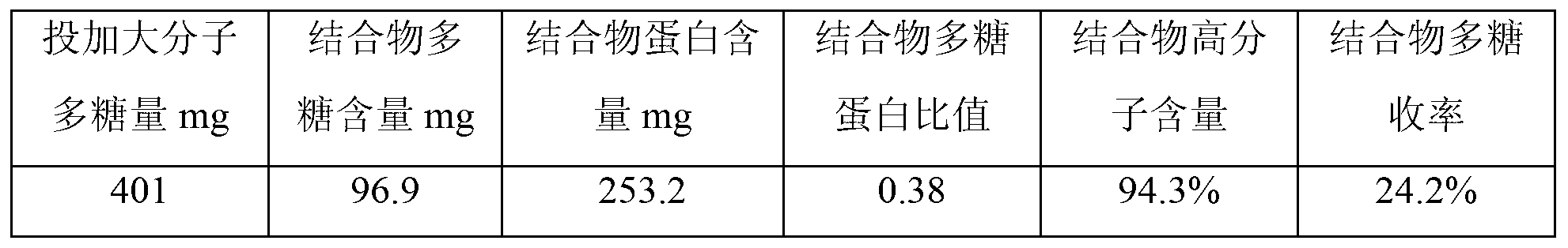
[0020] S1. Gel filtration chromatography purification of polysaccharides. Dilute the Hib capsular polysaccharides with water for injection to a solution with a concentration of 5 mg / ml, and filter them with a filter membrane with a pore size of 0.45 μm. The filtrate is purified by Sepharose4FF gel chromatography column chromatography. , load the sample on Sepharose4FF gel chromatography column with 10% column volume, elute with 0.15M sodium chloride as the eluent, collect the chromatographic eluate with KD≤0.3, and then use 300KD ultrafiltration to collect the eluate Concentrate by membrane ultrafiltration to a polysaccharide concentration of 6 mg / ml to obtain the macromolecular Hib capsular polysaccharide;
[0021] According to this method, macromolecular Hib capsular polysaccharides were prepared in total, and the preparation results were as follows...
Embodiment 2
[0029] A method for preparing Haemophilus influenzae type b polysaccharide conjugate vaccine, comprising the following steps:
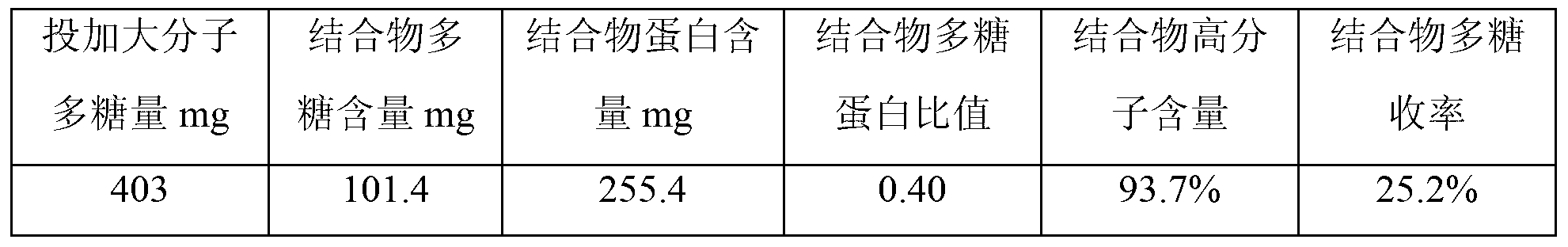
[0030] S1. Gel filtration chromatography purification of polysaccharides. Dilute the Hib capsular polysaccharides with water for injection to a solution with a concentration of 3 mg / ml, and filter them with a filter membrane with a pore size of 0.45 μm. The filtrate is purified by Sepharose4FF gel chromatography column chromatography. , load the sample on Sepharose4FF gel chromatography column with 8% column volume, elute with 0.15M sodium chloride as the eluent, collect the chromatographic eluate with KD≤0.3, and then use 300KD ultrafiltration to collect the eluate Concentrate by membrane ultrafiltration to a polysaccharide concentration of 4 mg / ml to obtain the macromolecular Hib capsular polysaccharide;
[0031] According to this method, macromolecular Hib capsular polysaccharides were prepared in total, and the preparation results were as follows:...
Embodiment 3
[0039] A method for preparing Haemophilus influenzae type b polysaccharide conjugate vaccine, comprising the following steps:
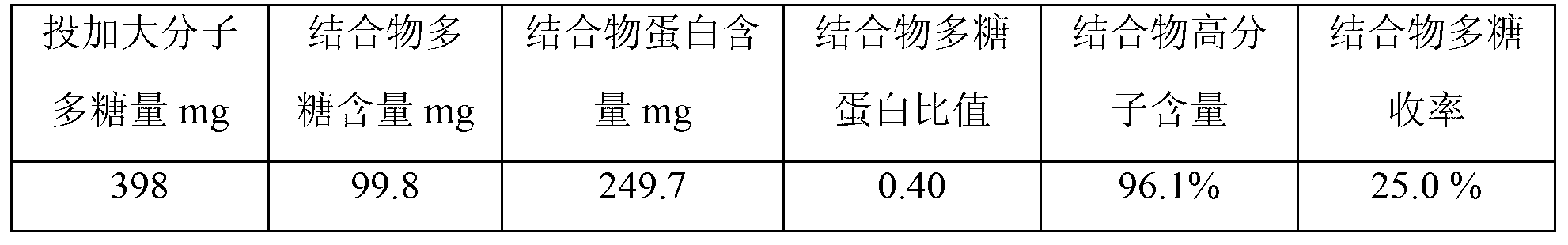
[0040] S1. Gel filtration chromatography purification of polysaccharides. Dilute the Hib capsular polysaccharides with water for injection to a solution with a concentration of 6 mg / ml, and filter them with a filter membrane with a pore size of 0.45 μm. The filtrate is purified by Sepharose4FF gel chromatography column chromatography. , load the sample on Sepharose4FF gel chromatography column with 5% column volume, elute with 0.15M sodium chloride as the eluent, collect the chromatographic eluate with KD≤0.3, and then use 300KD ultrafiltration to collect the eluate Concentrate by membrane ultrafiltration to a polysaccharide concentration of 5 mg / ml to obtain a macromolecular Hib capsular polysaccharide;
[0041] According to this method, macromolecular Hib capsular polysaccharides were prepared in total, and the preparation results were as follows: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com