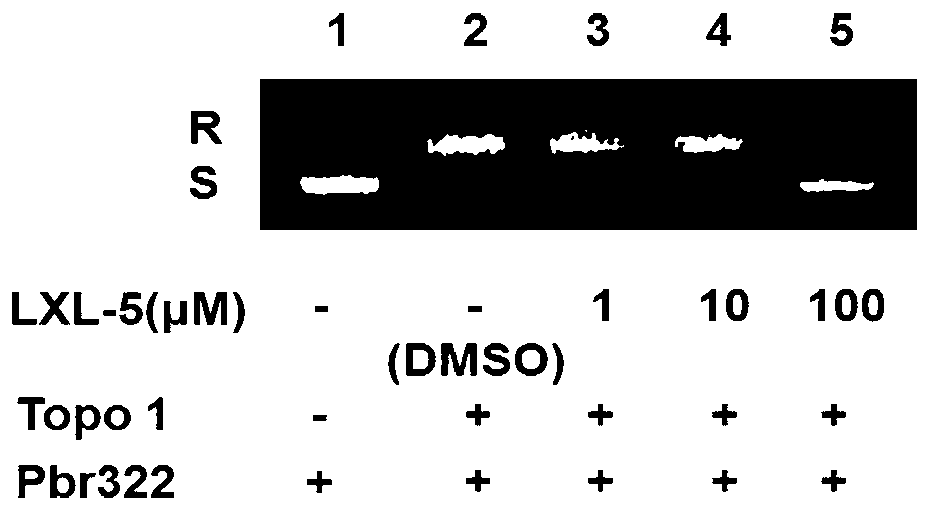
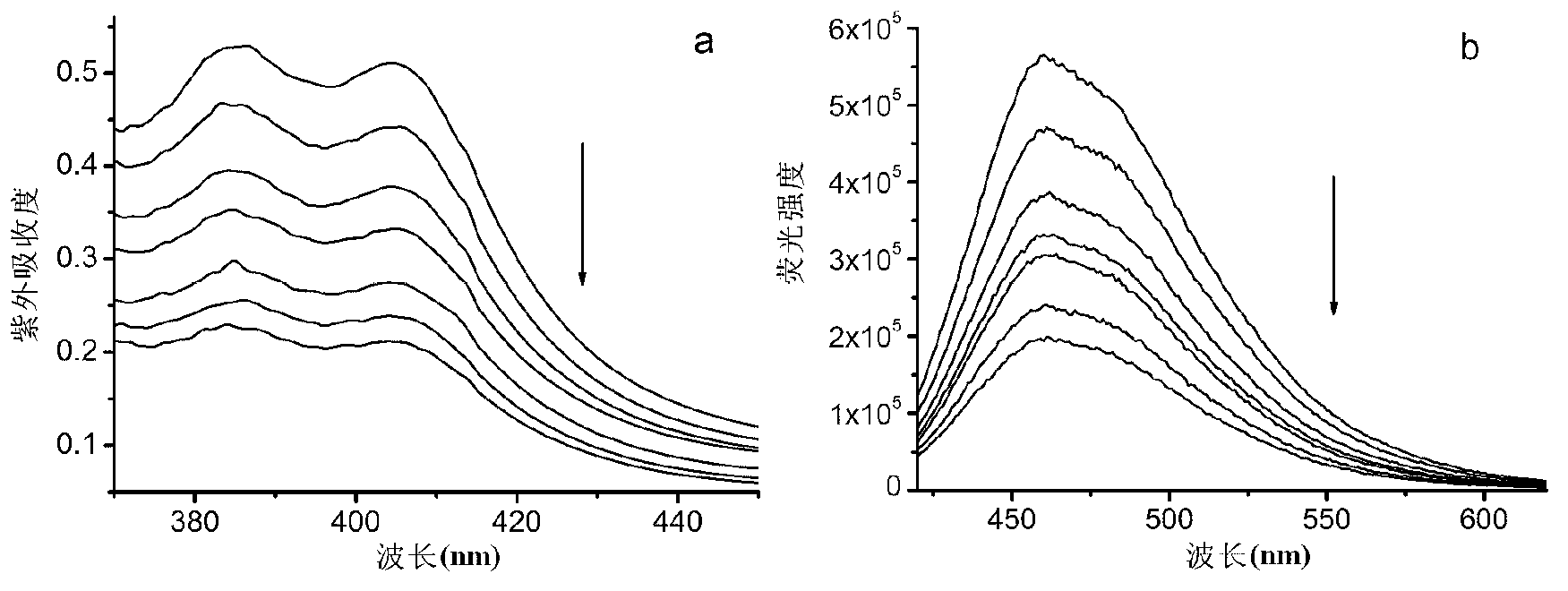
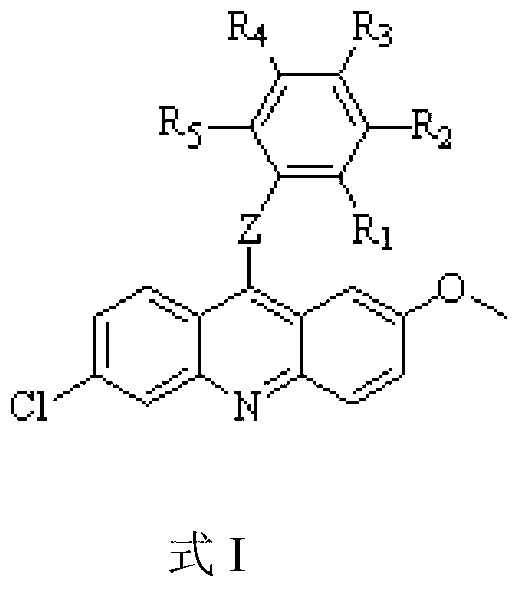
Acridine derivative and preparation method and application thereof
A drug and compound technology, applied in the field of 9-benzylamino (benzyloxy) acridine derivatives and its preparation, can solve the problems that acridine derivatives need to be strengthened
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1, preparation 4-chloro-2 (4-methoxy amino) benzoic acid
[0060]In dimethylformamide (DMF) (50.00ml) was added 2,4-dichlorobenzoic acid (2.00g, 10.47mmol), 4-methoxyaniline (0.86g, 6.99mmol), potassium carbonate (2.00g , 14.49mmol) and copper powder (0.20g, 3.15mmol). Stir overnight at 130°C. After the reaction mixture was cooled, it was added to 200 ml of water, and the pH value was adjusted to about 3 with acetic acid. After extraction with ethyl acetate, the organic phase was evaporated to dryness and separated by column chromatography to obtain 1.24 g of a yellow solid (compound represented by formula IV, 4-chloro-2(4-methoxyanilino)benzoic acid). Yield 63.9%; melting point 198-201°C.
[0061] The confirmed data of the compound structure are:
[0062] 1 H NMR (400MHz, CDCl 3 )δ9.16(s,1H),δ7.93(d,J=8.6Hz,1H),δ7.17(d,J=8.7Hz,2H),δ6.95(d,J=8.8Hz,2H ),δ6.87(d,J=1.8Hz,1H),δ6.64(dd,J=8.6,1.7Hz,1H),δ3.84(s,3H); 13 C NMR (100.6MHz, CDCl 3 ) δ 172.79, 15...
Embodiment 2
[0063] Embodiment 2, preparation 6,9-dichloro-2-methoxyacridine
[0064] The 4-chloro-2(4-methoxyanilino)benzoic acid (2.00g, 7.19mmol) prepared in Example 1 was added to anhydrous phosphorus oxychloride (25.00ml), and refluxed at 140°C for 3 hours . After the reaction solution was cooled, it was slowly added to a mixture of ice water, ammonia water and chloroform (volume ratio 1:1:1). Chloroform was separated, extracted with chloroform again, and all chloroform extracts were combined, evaporated to dryness, and dried. 1.98 g of a yellow solid (compound represented by formula V, 6,9-dichloro-2-methoxyacridine) was obtained. Yield 99%; melting point 160-163°C.
[0065] The confirmed data of the compound structure are:
[0066] 1 H NMR (400MHz, CDCl 3 )δ8.34(d,J=9.2Hz,1H),δ8.20(d,J=1.9Hz,1H),δ8.09(d,J=10.2Hz,1H),δ7.56(dd,J =9.2,2.0Hz,1H), δ7.54–7.48(m,2H), δ4.04(s,3H).
Embodiment 3
[0067] Embodiment 3, preparation 6-chloro-2-methoxy-9-benzylamino-acridine
[0068] Add 6,9-dichloro-2-methoxyacridine (50.00mg, 0.18mmol) and various amine compounds (0.97mmol) prepared in Example 2 into 10.00ml of absolute ethanol, add potassium carbonate, Potassium iodide, heated under reflux at 85-100 degrees Celsius and stirred overnight. The reaction solution was evaporated to dryness, extracted with ethyl acetate, and the organic phase was evaporated to dryness and separated by column chromatography to obtain the desired compound (compound shown in formula I):
[0069] 1) Preparation of 6-chloro-2-methoxy-9-(4-trifluoromethyl)benzylamino-acridine (R in formula I 4 =CF 3 , Z is NHCH 2 , and the rest of the substituents are all H)
[0070] Yield 81%; melting point 152-154 ° C; high resolution mass spectrum (ESI): calculated value [C 22 h 16 CIF 3 N 2 O+H] + 417.0981; experimental value: 417.0986.
[0071] The confirmed data of the compound structure are:
[007...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com