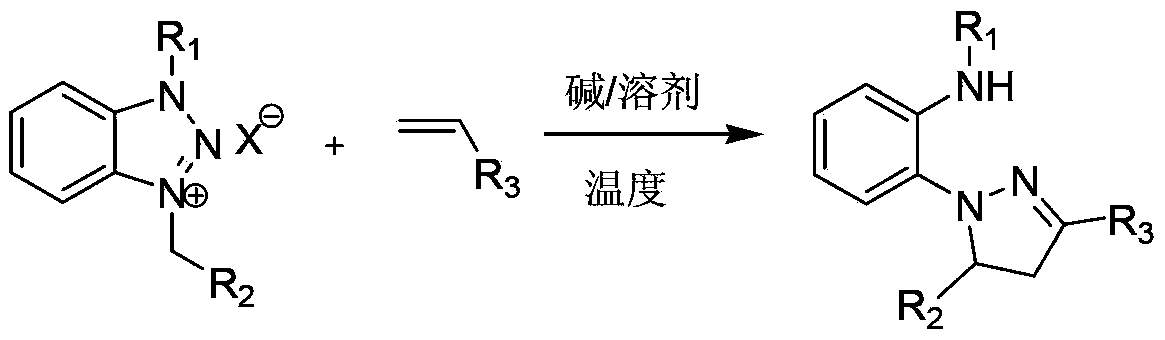
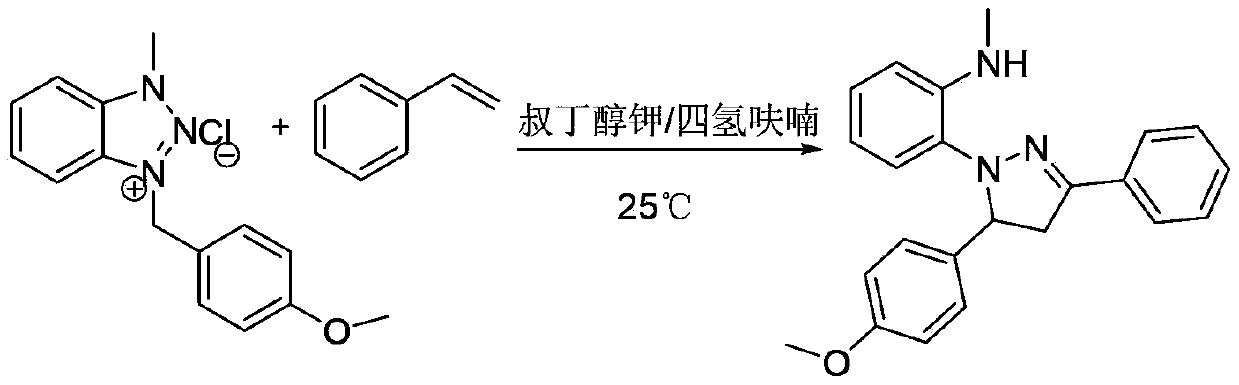
A method for preparing 1,3,5-triaryl-substituted pyrazoline derivatives
A pyrazoline and triaryl technology, which is applied in the field of organic chemical synthesis, can solve the problems of great harm to human body and environment, complicated operation process, difficult separation of products, etc., and achieves the effects of cheap raw materials, good selectivity, and overcoming high toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
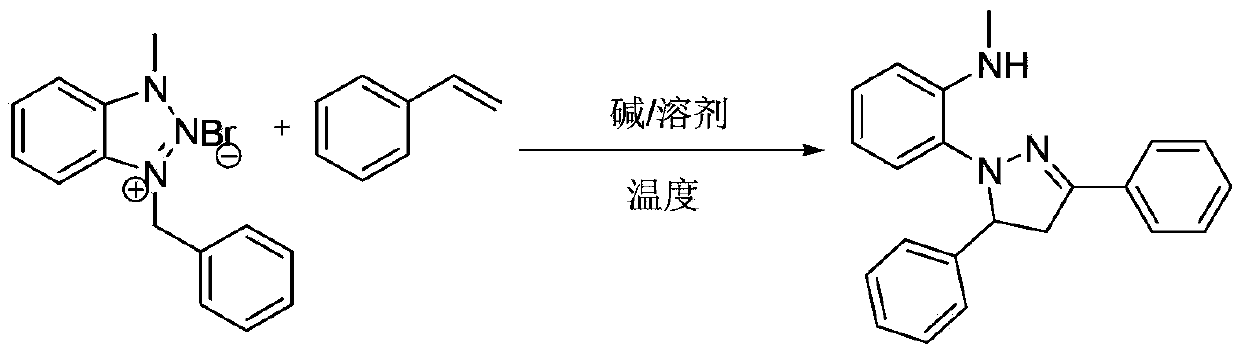
[0017] Example 1: Preparation of 2-(3,5-diphenyl-4,5-dihydropyrazolyl)-N-methylaniline
[0018] The reaction formula is:
[0019]
[0020] At room temperature, add 0.30g (1mmol) 1-methyl-3-benzylbenzotriazole bromide, 0.10g (1mmol) styrene, 25mL tetrahydrofuran (THF) to a 50ml round bottom flask, stir for 10 Minutes later, 0.11 g (1 mmol) of potassium tert-butoxide (t-BuOK) was added, followed by thin-layer chromatography (developing solvent: ethyl acetate:petroleum ether=1:5, volume ratio). After the reaction was completed, THF was concentrated under reduced pressure to recover THF, 10 ml of water and 20 ml of ethyl acetate were added to the residue, and the liquid was separated. Wash with saturated brine, and dry over anhydrous magnesium sulfate. Ethyl acetate was recovered by concentration under reduced pressure, and the residue was quickly separated by column chromatography (eluent: ethyl acetate: petroleum ether = 1:15, volume ratio) to obtain 0.21 g of a yellow soli...
Embodiment 2
[0021] Example 2: Preparation of 2-(3,5-diphenyl-4,5-dihydropyrazolyl)-N-methylaniline
[0022] At room temperature, add 0.30 g (1 mmol) of 1-methyl-3-benzylbenzotriazole bromide, 0.10 g (1 mmol) of styrene, 25 mL of acetonitrile (CH 3 CN), after stirring for 10 minutes, add 0.11g (1mmol) potassium tert-butoxide (t-BuOK), and trace with thin-layer chromatography (developing solvent: ethyl acetate:petroleum ether=1:5, volume ratio). After the reaction is over, concentrate under reduced pressure to recover CH 3 CN, add 10 milliliters of water and 20 milliliters of ethyl acetate to the residue, separate the layers, extract the organic phase with 2×20 milliliters of ethyl acetate, combine the organic layers, wash with 2×20 milliliters of saturated brine, wash with anhydrous magnesium sulfate to dry. Ethyl acetate was recovered by concentration under reduced pressure, and the residue was quickly separated by column chromatography (eluent: ethyl acetate: petroleum ether = 1:15, vo...
Embodiment 3
[0023] Example 3: Preparation of 2-(3,5-diphenyl-4,5-dihydropyrazolyl)-N-methylaniline
[0024] At room temperature, add 0.30g (1mmol) 1-methyl-3-benzylbenzotriazole bromide, 0.10g (1mmol) styrene, 25mL tetrahydrofuran (THF) to a 50ml round bottom flask, stir for 10 Minutes later, 0.04 g (1 mmol) of sodium hydride (NaH) was added, followed by thin-layer chromatography (developing solvent: ethyl acetate:petroleum ether=1:5, volume ratio). After the reaction was completed, THF was concentrated under reduced pressure to recover THF, 10 ml of water and 20 ml of ethyl acetate were added to the residue, and the liquid was separated. Wash with saturated brine, and dry over anhydrous magnesium sulfate. Ethyl acetate was recovered by concentration under reduced pressure, and the residue was quickly separated by column chromatography (eluent: ethyl acetate: petroleum ether = 1:15, volume ratio) to obtain 0.16 g of a yellow solid product with a yield of 49.5%. Test data is with embodim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com