Compound with antihypertensive activity and preparation method thereof
A compound and high blood pressure technology, applied in the field of biomedicine, can solve the problems of toxic and side effects of chemical drugs, chemical drug treatment can not meet expectations, etc., achieve the effect of cheap and easy to obtain reagents, mild reaction conditions, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
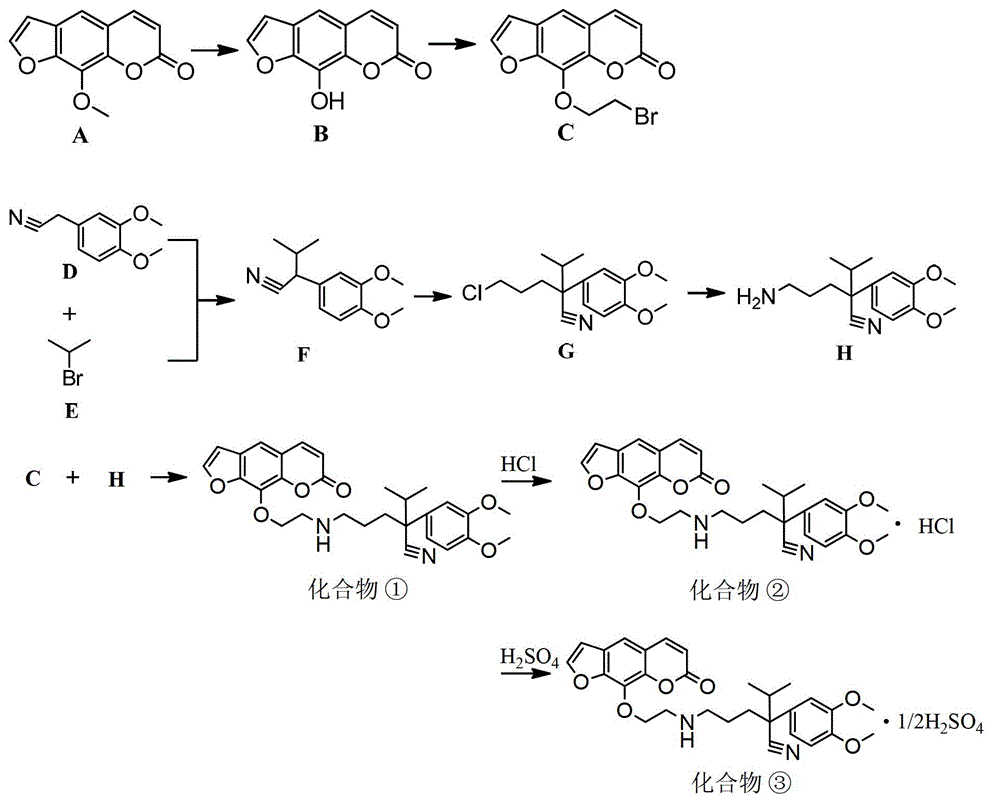
[0042] Example 1 9-[[α-[3-(2-ethyl)amino]propyl]-3,4-dimethoxy-α-(1-methylethyl)phenylacetonitrileoxy]-7H-furan [3,2-g]chromen-7-one and its hydrochloride, prepared by the following steps:
[0043] 1) Demethylation of compound A 8-methoxypsoralen by boron tribromide to obtain compound B xanthoxylin
[0044] Weigh 5.00g (24.75mmol) of 8-methoxypsoralen into a 250mL round-bottomed flask, stir and dissolve with 70mL of anhydrous dichloromethane, under nitrogen protection, stir for 10min under ice-salt bath conditions, and dissolve 13mL of three Dilute the boron bromide (2N) solution into 20mL of anhydrous dichloromethane, and then slowly add it dropwise into the dissolved 8-methoxypsoralen solution in the dark, the dropping time is controlled at 20min, and then placed at room temperature After reacting for 6 hours, neutralize with alkaline solution after the reaction, then filter and dry to obtain 4.30 g (21.29 mmol) of white solid powder with a yield of 92.1%.
[0045] Its phy...
Embodiment 2
[0064] Example 2 9-[[α-[3-(2-ethyl)amino]propyl]-3,4-dimethoxy-α-(1-methylethyl)phenylacetonitrileoxy]-7H-furan [3,2-g]chromen-7-one and its sulfate, prepared by:
[0065] Step 1) to step 6) are the same as in Example 1, that is, from compound A 8-methoxypsoralen to compound ① 9-[[α-[3-(2-ethyl)amino]propyl]-3 , The preparation steps of 4-dimethoxy-α-(1-methylethyl) phenylacetonitrile oxy]-7H-fur[3,2-g]chromen-7-one are the same; compound ③9-[[α -[3-(2-Ethyl)amino]propyl]-3,4-dimethoxy-α-(1-methylethyl)phenylacetonitrileoxy]-7H-furano[3,2-g]color The preparation of en-7-one sulfate, specifically:
[0066] 7) Compound ①9-[[α-[3-(2-ethyl)amino]propyl]-3,4-dimethoxy-α-(1-methylethyl)phenylacetonitrileoxy]-7H-furan [3,2-g] Chromene-7-one reacts with sulfuric acid to obtain compound ③ 9-[[α-[3-(2-ethyl)amino]propyl]-3,4-dimethoxy-α- (1-Methylethyl)phenylacetonitrileoxy]-7H-furo[3,2-g]chromen-7-one sulfate
[0067] Weigh 4.00g (7.94mmol) of 9-[[α-[3-(2-ethyl)amino]propyl]-3,4-d...
Embodiment 3
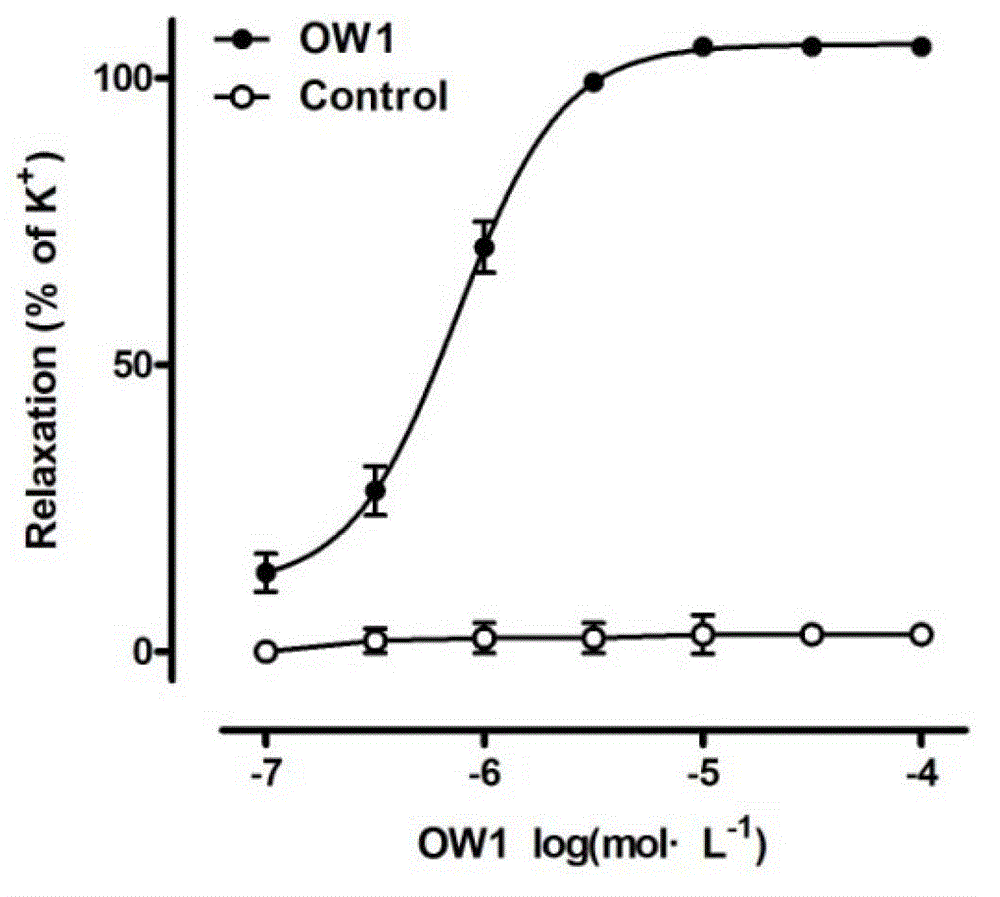
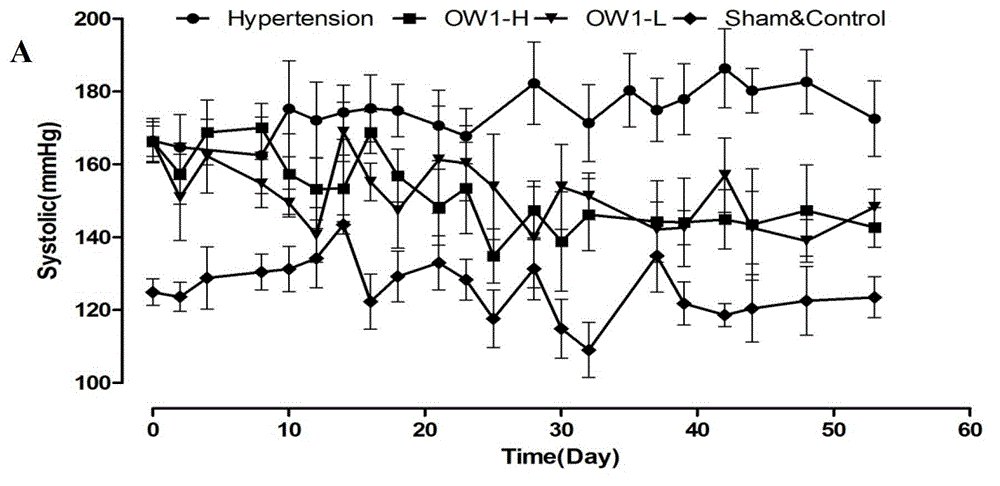
[0069] Example 3 9-[[α-[3-(2-ethyl)amino]propyl]-3,4-dimethoxy-α-(1-methylethyl)phenylacetonitrileoxy]-7H-furan [3,2-g]chromen-7-one (OW1) relaxes mesenteric artery in rats
[0070] Male SD rats (body weight 200-300 g, Experimental Animal Center, Xi'an Jiaotong University Medical College) were killed by neck dislocation, and the secondary branches of the mesenteric artery (MA) were taken out and placed in pre-cooled Krebs solution (composition (g / L): 6.954 NaCl, 0.343KCl, 1.260NaHCO3, 0.187NaH2PO4·2H2O, 0.166CaCl2, 0.244MgCl2·6H2O, 1.090 glucose, PH=7.2~7.4), and make a vascular ring specimen with a length of 3 mm. The vascular ring specimens were connected with a DMT vascular tension measurement system (Danish Myo Technology AIS Inc. 610M) with thin steel wires to record changes in vascular tension. The vascular ring specimens were balanced in Krebs solution saturated with 95% oxygen 5% carbon dioxide gas mixture at 37°C, and adjusted to zero until the vascular tension did n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com