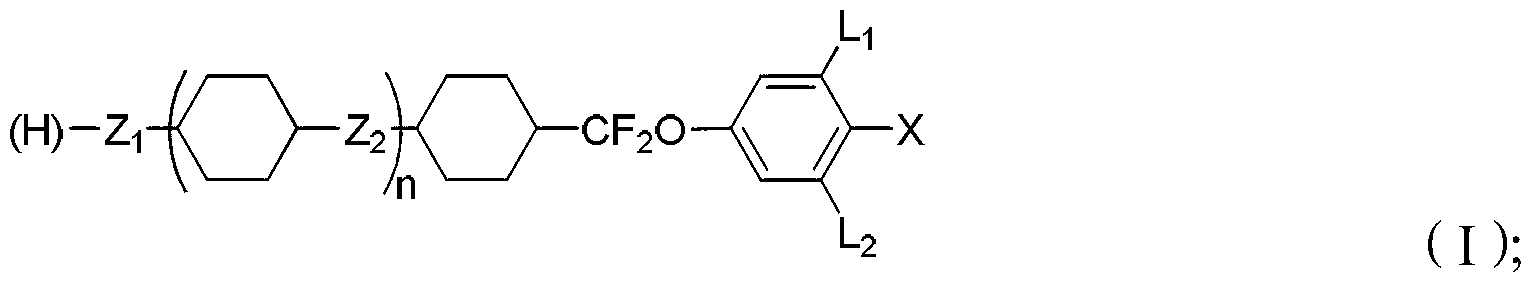
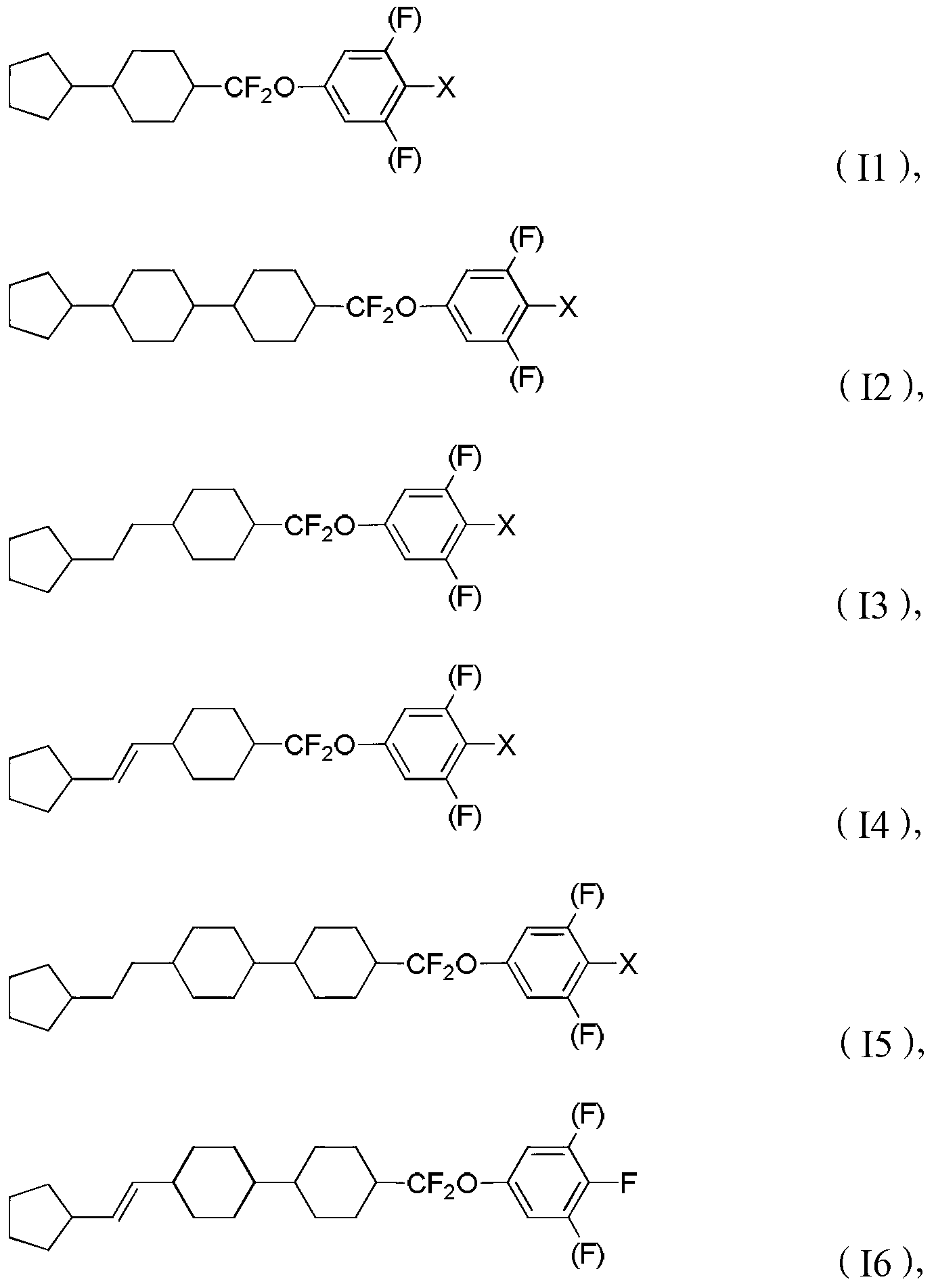
Liquid crystal compound containing difluoro-methylene key bridge, preparation method thereof and composition containing liquid crystal compound
A difluoromethylene bond bridge and liquid crystal compound technology, applied in the field of liquid crystal compounds, can solve the problems of TFT-LCD such as insufficient response speed, low voltage, and insufficient charge retention rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] compound The synthetic route of (I-A) is shown in the following formula, and its specific preparation steps are as follows:
[0070]
[0071] Synthesis of Step 1.1 (I-A-a)
[0072] Add 1 mol cyclopentane bromide and 0.9 mol triphenylphosphine into a 1 L three-necked flask, then raise the temperature to 140° C., stir and react at constant temperature for 6 h, and solids precipitate out during the reaction. After completion of the reaction, filter and wash the obtained filter cake with toluene to obtain 0.76 mol of bromocyclopentane triphenylphosphine salt (I-A-a) with a yield of 84.4%.
[0073] Synthesis of Step 1.2 (I-A-b)
[0074] Add 0.25mol of (I-A-a) and 250mL of dry tetrahydrofuran to a 1L three-necked flask. After the addition is completely dissolved, blow nitrogen into the three-necked flask for nitrogen protection, and then use an ice-salt bath to cool the solution in the three-necked flask to 0°C; Add 0.25mol potassium tert-butoxide into the above-mentio...
Embodiment 2
[0093] preparation The synthetic route of (I-B) is shown in the following formula, and its specific preparation steps are as follows:
[0094]
[0095] Synthesis of Step 2.1 (I-B-a)
[0096] Add 1mol cyclopentylmethanol, 1.2mol iodine, 1.2mol imidazole and 500mL toluene into a 2L three-necked flask, stir and heat to raise the temperature of the solution in the three-necked flask to 25°C-30°C, and react with constant temperature stirring for 6h; The obtained reaction product was subjected to conventional post-treatment to obtain 0.74 mol of the product cyclopentylmethyl iodide (I-B-a), with a yield of 74%, GC: 96%.
[0097] Synthesis of Step 2.2 (I-B-b)
[0098] Add 0.74mol cyclopentylmethyl iodide (I-B-a), 1mol triphenylphosphine and 500mL toluene to a 2L three-necked flask, heat the solution in the three-necked flask to reflux, and react at a constant temperature at the reflux temperature for 4 hours; The product was filtered, and the obtained filter cake was washed wi...
Embodiment 3
[0114] preparation The synthetic route of (I-C) is shown in the following formula, and the specific preparation steps are as follows:
[0115]
[0116] Synthesis of Step 3.1 (I-C-a)
[0117] Add 0.1mol (I-B-e), 0.5g of palladium carbon and 300mL of absolute ethanol to a 1L one-necked bottle, and then pass hydrogen into the three-necked bottle for air exhausting, repeating 5 times; after the air in the three-necked bottle is exhausted, carry out shock hydrogenation at room temperature Operation, the hydrogenation time is 6h; after the reaction is completed, the reaction product is filtered, and the resulting filter cake is subjected to conventional post-treatment to obtain (I-C-a) 0.95mol, the yield is 95%, GC: 97.7%.
[0118] Synthesis of Step 3.2 (I-C-b)
[0119] (I-C-b) was synthesized referring to Step 2.6 of Example 2.
[0120] Synthesis of Step 3.3 (I-C)
[0121] (I-C) was synthesized referring to Step 1.8 of Example 1.
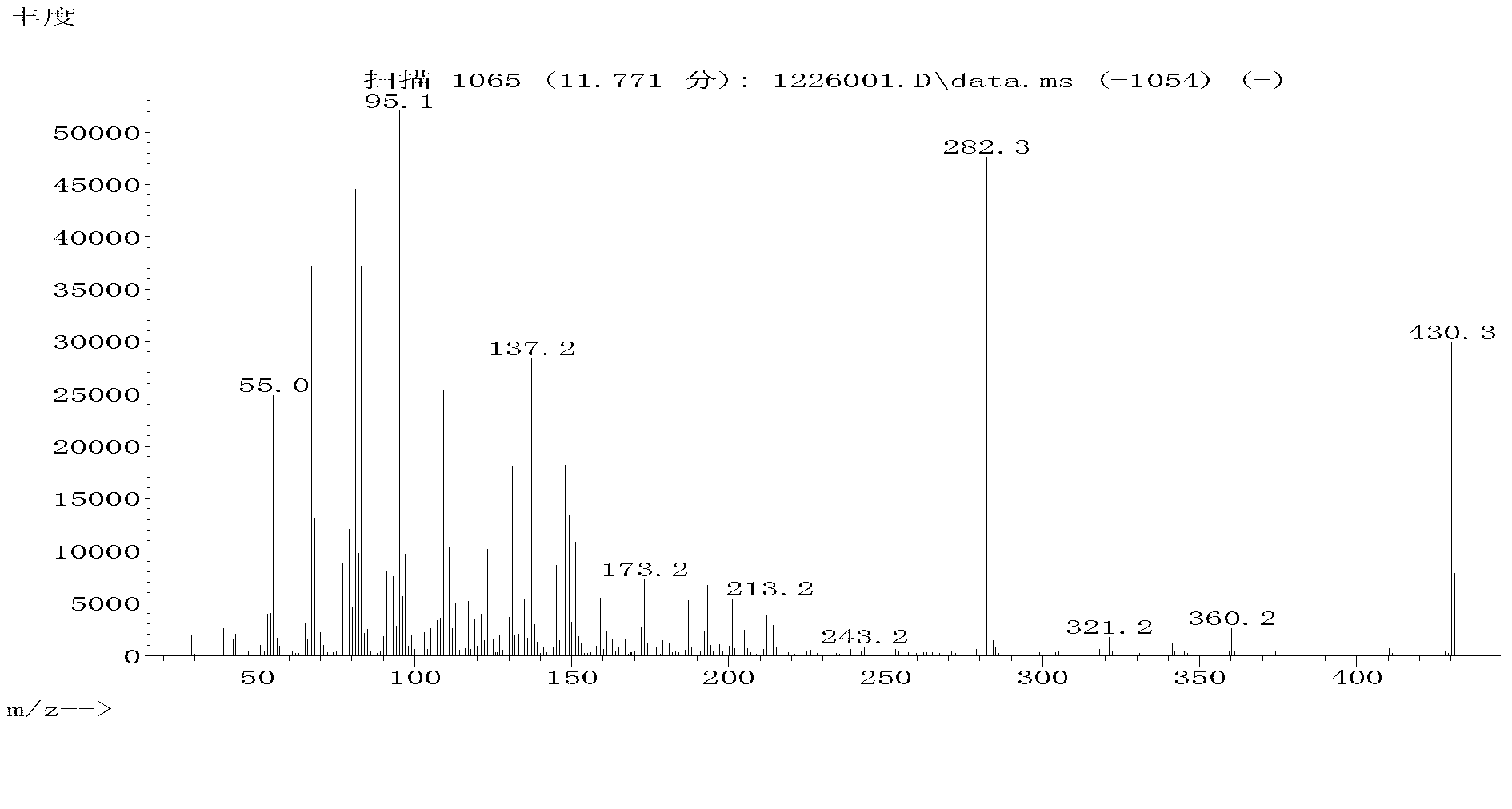
[0122] The (I-C) prepared in Example 3 wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com