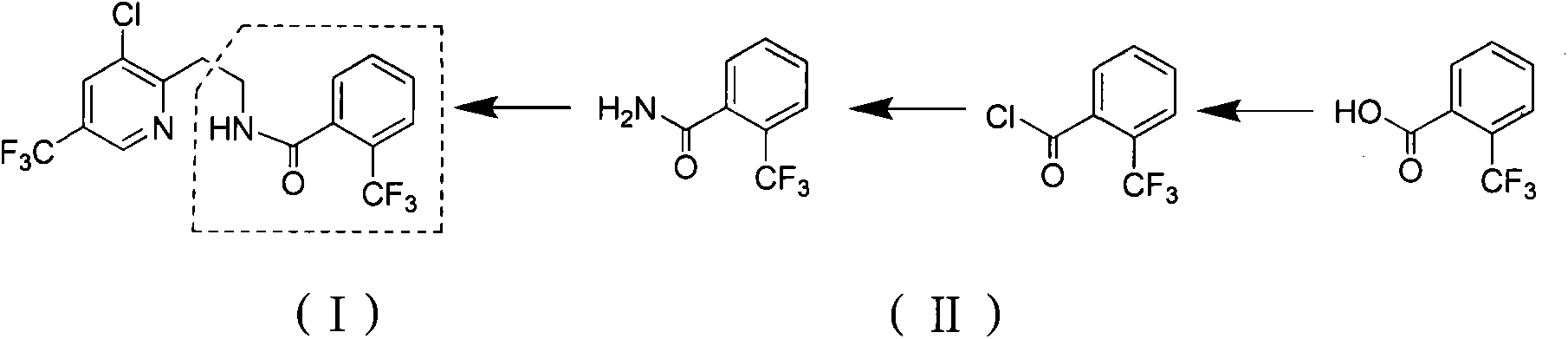
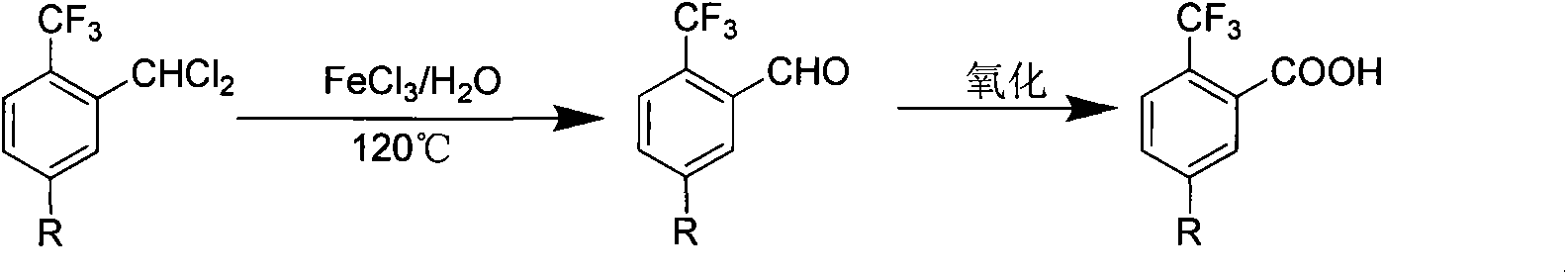
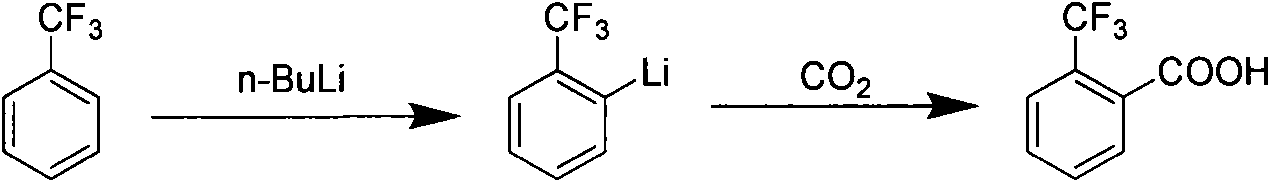
Preparation method of 2-trifluoromethyl benzoic acid
A technology of trifluoromethylbenzoic acid and trifluoromethyldichlorobenzyl, which is applied in the field of preparation of 2-trifluoromethylbenzoic acid, can solve problems such as expensive, harsh reaction conditions, harmful to human body and environment, and achieve catalyst Less dosage, high fluorination yield and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: In a 5L stainless steel autoclave equipped with a stirrer and a thermometer, cool down to below 5°C, add hydrogen fluoride and 2-trichloromethyl benzyl dichloride in sequence, and the mass ratio of the two is 0.2:1, and then Add the catalyst perfluorooctanesulfonyl fluoride, the mass ratio of 2-trichloromethyl benzyl dichloride to the catalyst is 1:0.001, raise the temperature to 80-90°C, control the reaction pressure to 2.5-2.8MPa, and react for 4 hours. Sampling and GC detection showed that the content of the intermediate product 2-difluorochloromethyl benzyl dichloride was 0.3%. After the reaction is completed, excess hydrogen fluoride is removed by purging with nitrogen, and neutralized to pH=6-7 with potassium carbonate aqueous solution. After standing still, the product 2-trifluoromethyl benzyl dichloride was isolated with a content of 96.6% and a yield of 94.1%.
[0052] The reaction formula for fluorination is:
[0053]
[0054] The fluorinati...
Embodiment 2
[0059]Embodiment two: in the 5L stainless steel autoclave that is equipped with stirrer, thermometer, cool down to below 5 ℃, add hydrogen fluoride and 2-trichloromethyl benzyl dichloride successively, and the mass ratio of the two is 1: 1, then Add the catalyst perfluorobutylsulfonyl fluoride, the amount of 2-trichloromethyl benzyl dichloride and the catalyst is 1:0.01, raise the temperature to 50-60°C, control the reaction pressure 0.8-1.2MPa, and react for 4 hours. Sampling and GC detection showed that the content of the intermediate product 2-difluorochloromethyl benzyl dichloride was 0.2%. After the reaction is completed, excess hydrogen fluoride is removed by purging with nitrogen, and neutralized to pH=6-7 with potassium carbonate aqueous solution. After standing still, the product 2-trifluoromethyl benzyl dichloride was isolated with a content of 97.5% and a yield of 93.5%.
[0060] The reaction formula for fluorination is:
[0061]
[0062] The fluorination react...
Embodiment 3
[0067] Embodiment three: in the 5L stainless steel autoclave that is equipped with stirrer, thermometer, cool down to below 5 ℃, add hydrogen fluoride and 2-trichloromethyl benzyl dichloride successively, and the mass ratio of the two is 0.5: 1, then Add a catalyst, the catalyst is composed of a mixture of perfluoropentylsulfonyl fluoride, perfluoroheptylsulfonyl fluoride and perfluorooctylsulfonyl fluoride, the amount of 2-trichloromethyl benzyl dichloride and the catalyst is 1:0.005, and the temperature rises To 140-150°C, control the reaction pressure to 4.5-5.0MPa, and react for 4 hours. Sampling and GC detection showed that the content of the intermediate product 2-difluorochloromethyl benzyl dichloride was 0.5%. After the reaction is completed, excess hydrogen fluoride is removed by purging with nitrogen, and neutralized to pH=6-7 with potassium carbonate aqueous solution. After standing still, the product 2-trifluoromethyl benzyl dichloride was isolated with a content ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com