Preparation method of Esomeprazole and preparation method of Esomeprazole sodium
A technology of esomeprazole sodium and esomeprazole, which is applied in the field of medicine, can solve the problems of cumbersome preparation process, achieve good stereoselectivity and avoid the effect of generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
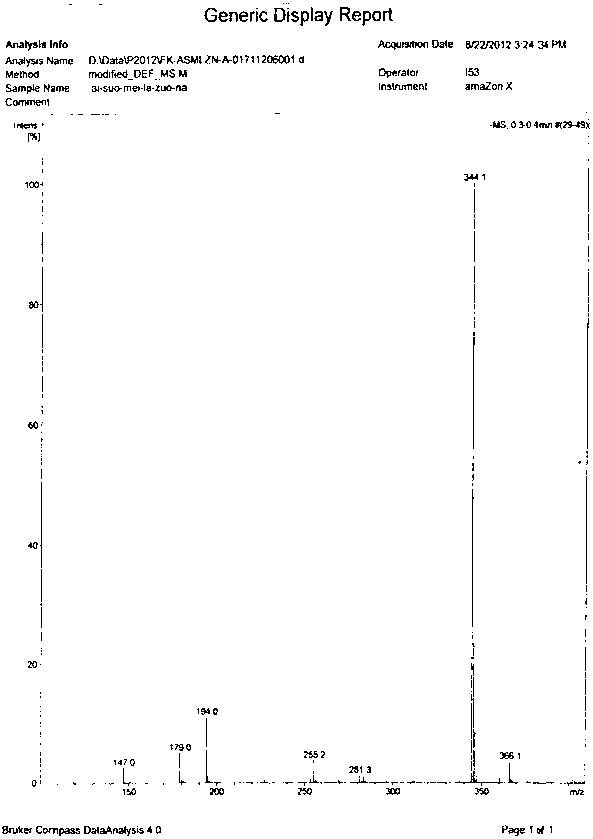
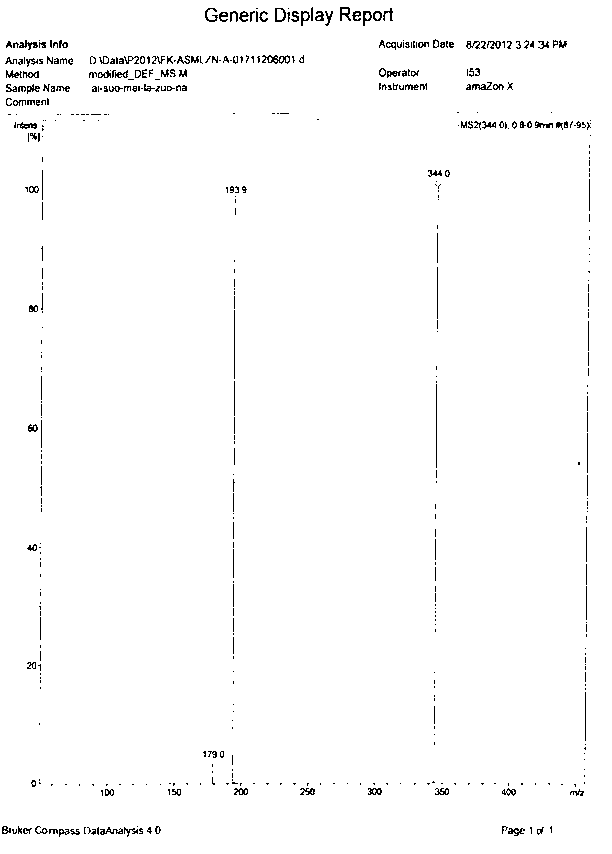
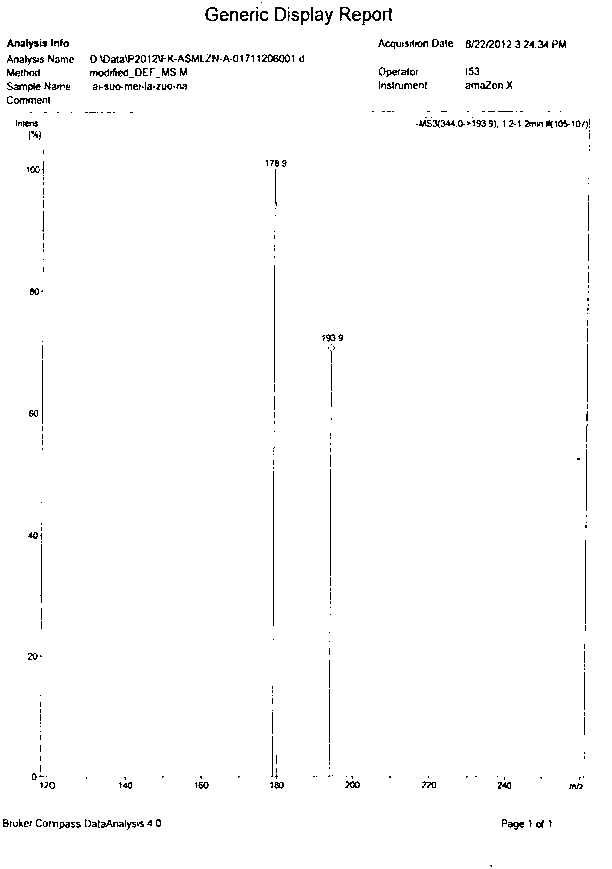
Image
Examples
preparation example Construction
[0043] The invention provides a kind of preparation method of esomeprazole, comprising:
[0044]5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridyl)methyl)thio)-1H-benzimidazole, (1S,2S)- (-)-1,2-cyclohexanediamine-D-tartrate, cobalt naphthenate and water are added to an organic solvent for reaction to obtain a reaction solution;
[0045] Oxidant and water are added into the reaction liquid, and esomeprazole is obtained after reaction.
[0046] The present invention first adds 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridyl)methyl)thio)-1H-benzimidazole into the organic solvent , and heat up to dissolve it to obtain a clear solution; the organic solvent is preferably ethyl acetate, toluene or acetone, more preferably ethyl acetate; the temperature of dissolution can be 60°C to 75°C, preferably 62°C to 68°C ℃.
[0047] Cool the clear solution, add (1S,2S)-(-)-1,2-cyclohexanediamine-D-tartrate, cobalt naphthenate and water, and stir to obtain a reaction solution; the (1S, 2S)-(-...
Embodiment 1
[0079] 15 g (45 mmol) of 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridyl)methyl)thio)-1H-benzimidazole Add 75ml of ethyl acetate, heat up to 65°C, after dissolving, lower the temperature to 55°C, add 11.89g (45mmol) (1S,2S)-(-)-1,2-cyclohexanediamine- D-tartrate and 14.09 g (45 mmol) cobalt naphthenate, add a small amount of purified water at the same time, stir for 2 hours, lower the temperature to 0-5 ° C, add 2 ml of water at this temperature, and use 2-3 hours to dissolve 3.55 g (45 mmol) of sodium peroxide was slowly added to the reaction solution. After the addition was complete, the reaction was carried out at 0-5° C. for 2 hours, and the end point of the reaction was determined by TLC. After the reaction was completed, the solid was obtained by filtration, washed with petroleum ether, and dried to obtain 13.4 g of esomeprazole sodium, with a yield of 80%.
[0080] Through content determination of the esomeprazole sodium, the content is ≥ 98.0%, and the single compound...
Embodiment 2
[0100] 15 g (45 mmol) of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfanyl]-1H-benzimidazole Add 75ml of ethyl acetate, heat up to 65°C, after dissolving, lower the temperature to 55°C, add 5.28g (20mmol) (1S,2S)-(-)-1,2-cyclohexanediamine- D-tartrate and 6.26 g (20 mmol) cobalt naphthenate, add a small amount of purified water at the same time, stir for 2 hours, lower the temperature to 0-5 ° C, add 2 ml of water at this temperature, and use 2-3 hours to dissolve 3.55 g (45 mmol) of sodium peroxide was slowly added to the reaction solution. After the addition was complete, the reaction was carried out at 0-5° C. for 2 hours, and the end point of the reaction was determined by TLC. After the reaction was completed, the solid was obtained by filtration, washed with petroleum ether, and dried to obtain 13.4 g of esomeprazole sodium, with a yield of 80%.
[0101] Through content determination of the esomeprazole sodium, the content is ≥ 98.0%, and the single compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com