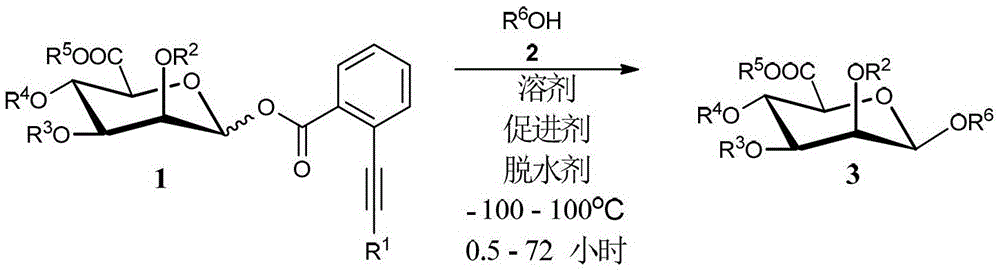
Synthetic method of β-d-mannuronic acid oligosaccharide or glycoside
A technology for the synthesis of uronic acid oligosaccharides and methods, applied in the field of organic synthesis, can solve the problems of harsh reaction conditions, difficult control, cumbersome operation, etc., and achieve the effects of high reaction yield, simple operation, and good stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
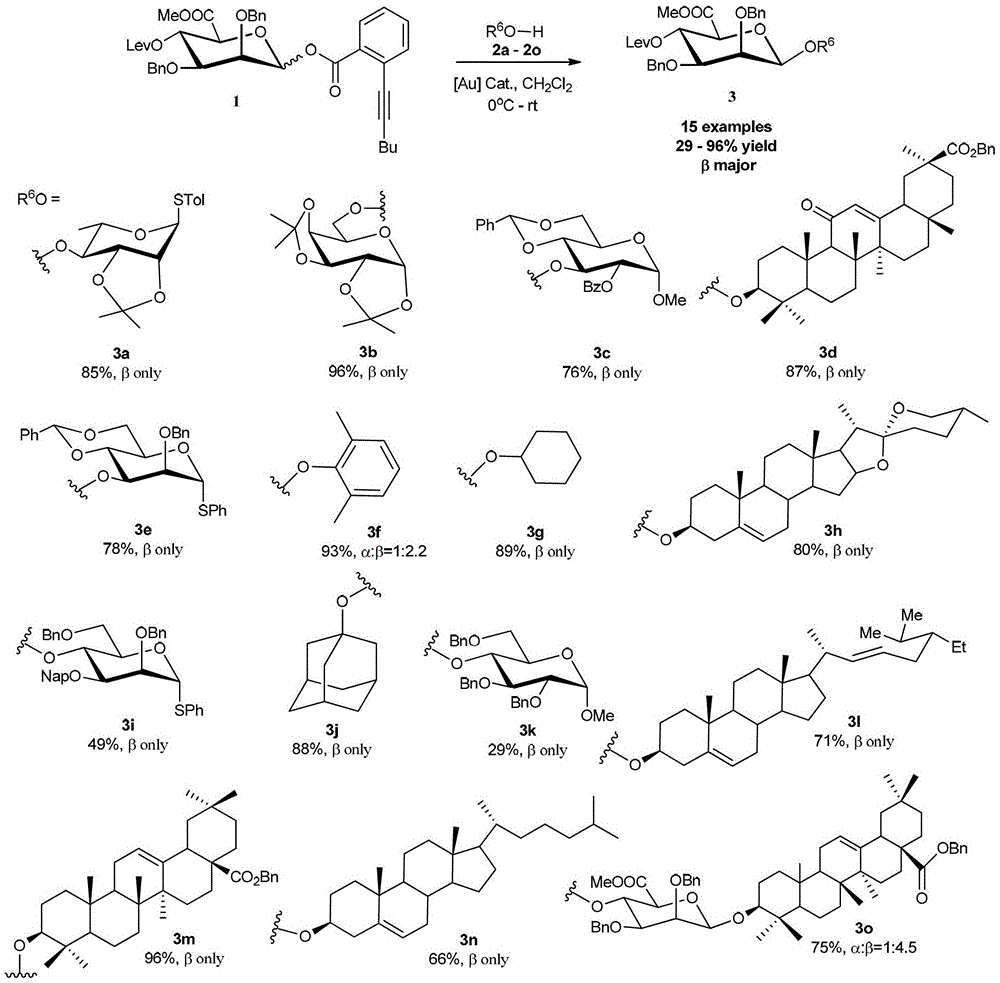
[0031] Embodiment 1-9: the synthesis of compound 3a, 3b, 3c, 3e, 3f, 3g, 3i, 3j, 3k
[0032] Under argon protection, add fresh activated AW-300 or Molecular sieves (3g / mmol), D-mannuronic acid o-alkynyl benzoate 1 (1.2-1.5mol), monosaccharides or alcohols or phenols 2a, 2b, 2c, 2e, 2f, 2g, 2i, 2j, One of 2k (1.0mol) and AgB (C 6 f 5 ) 4 (0.1mol), followed by injection of freshly distilled anhydrous CH 2 Cl 2 (2mL), after stirring at 0°C for 30min, add (4-MeOPh) 3 PAuCl (0.1mol) and AgB (C 6 f 5 ) 4 (0.1mol), continue to stir for 0.5-24 hours after TLC detects that the reaction is complete. The reaction was quenched with triethylamine and filtered, the filtrate was concentrated under reduced pressure, and column chromatography gave compounds 3a, 3b, 3c, 3e, 3f, 3g, 3i, 3j, 3k, respectively.
Embodiment 10-12
[0033] Embodiment 10-12: the synthesis of compound 3h, 3l, 3n
[0034] Similar to the steps of Example 1-9, with Ph 3 PAuCl (0.1mol) and AgB (C 6 f 5 )4 (0.1mol) as accelerator, D-mannuronic acid o-alkynyl benzoate 1 reacts with steroidal saponin 2h, 2l, 2n respectively to obtain 3h, 3l, 3n.
Embodiment 13-15
[0035] Embodiment 13-15: the synthesis of compound 3d, 3m, 3o
[0036] Similar to the steps of Example 1-9, with Me 3 PAuCl (0.1mol) and AgB (C 6 f 5 ) 4 (0.1mol) as accelerator, D-mannuronic acid o-alkynyl benzoate 1 reacts with one of triterpenoid saponin 2d, 2m, and triterpenoid saponin 2o respectively to obtain 3d, 3m, and 3o.
[0037] The data for compounds 3a-3o are as follows:
[0038] Compound 3a data:
[0039] Yield: 85%;
[0040] [α] D 20 -134.0 (c2.33, CHCl 3 );
[0041] 1 H NMR (600MHz, CDCl 3 )δ7.43(d,J=6.6Hz,2H),7.37(d,J=7.7Hz,2H),7.35-7.29(m,6H),7.26(m,4H),7.14(d,J=8.3 Hz,2H),5.65(s,1H),5.50(t,J=9.9Hz,1H),4.93(d,J=13.2Hz,2H),4.80(d,J=12.1Hz,1H),4.50( d,J=12.7Hz,1H),4.38(d,J=12.1Hz,1H),4.30(d,J=5.5Hz,1H),4.17-4.11(m,2H),3.94(d,J=2.8 Hz,1H),3.79(d,J=9.8Hz,1H),3.75(dd,J=9.9,7.7Hz,1H),3.70(s,3H),3.51(dd,J=9.9,3.3Hz,1H ),2.71(t,J=7.1Hz,2H),2.54(td,J=6.7,4.5Hz,2H),2.34(s,3H),2.16(s,3H),1.47(s,3H),1.34 (s,3H),1.30(d,J=6.2Hz,3H);
[0042] 13 C NMR (150...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com