Posaconazole freeze-dried powder injection and preparation method thereof
A freeze-dried powder injection and posaconazole technology, applied in the field of pharmaceutical preparations, can solve the problems of low posaconazole concentration, inconvenient transportation and storage, and poor stability, and achieve plump appearance, convenient transportation and storage, and stable performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
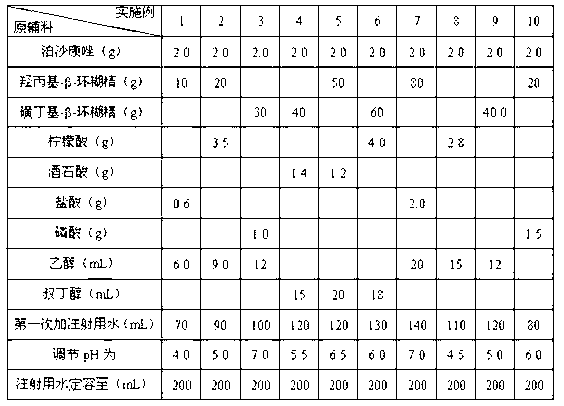
[0021] Prepare the liquid medicine of posaconazole freeze-dried powder injection according to the following dosage:
[0022]
Embodiment 11
[0026] prescription:
[0027] Posaconazole 20 g
[0028] Hydroxypropyl-β-cyclodextrin 200 g
[0029] Citric acid 35 g
[0030] Ethanol 90 mL
[0031] Add water for injection to 2000 mL
[0032] —————————————————
[0033] A total of 80 pieces were made
[0034] Preparation method: mix posaconazole and citric acid, add ethanol, heat to 60°C, and stir until uniform; add cyclodextrin and 900 mL of water for injection and stir to dissolve, then pour into the above solution, and stir until clear; Adjust the pH of the solution to 5.0, add water for injection to constant volume; depyrogenate, sterile filter, subpackage, and freeze-dry to obtain the product.
[0035]
Embodiment 12
[0037] Prescription:
[0038] Posaconazole 20 g
[0039] Sulfobutyl-β-cyclodextrin 400 g
[0040] Tartaric acid 14 g
[0041] tert-Butanol 150 mL
[0042] Add water for injection to 2000 mL
[0043] —————————————————
[0044] A total of 80 pieces were made
[0045] Preparation method: Stir and mix posaconazole and tartaric acid, add tert-butanol, and ultrasonicate to form a uniform state; take another cyclodextrin and add 1200 mL of water for injection, stir and dissolve, then pour into the above solution, and stir until clear; adjust the pH of the solution 5.5, add water for injection to constant volume; depyrogenation, sterile filtration, subpackaging, and freeze-drying.
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com