Method for preparing monohydric alcohol or dihydric alcohol through organic acid hydrogenation
An organic acid and monohydric alcohol technology, applied in the preparation of organic compounds, organic chemistry, chemical instruments and methods, etc., can solve the problems of difficult product separation and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0021] Preparation of Catalyst by Impregnation Method
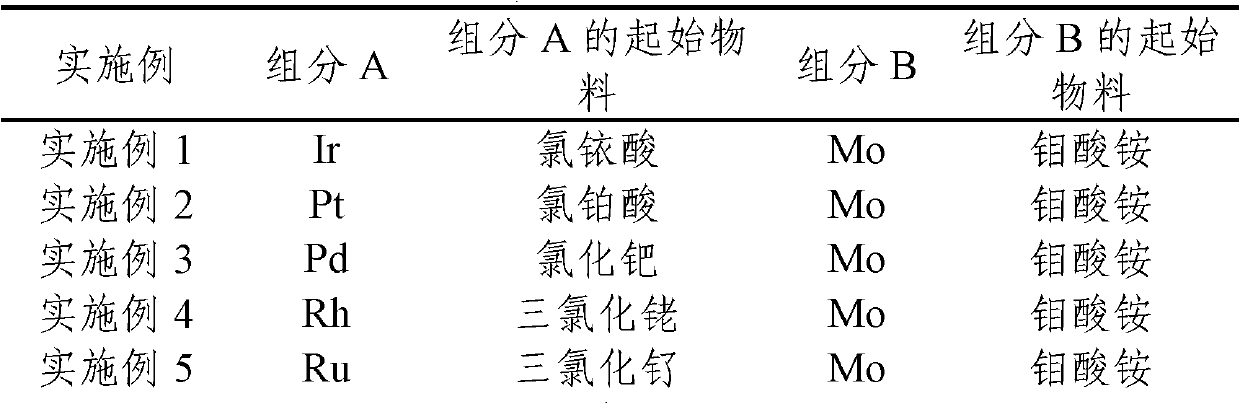
[0022] A soluble salt solution containing 10% (mass fraction) of A is added to the carrier for impregnation according to a certain metering ratio, aged at room temperature for 12 hours, and then dried in an oven at 120°C for 12 hours; then according to a certain B / A molar ratio Weigh the soluble salt solution of component B, add it to the above-mentioned carrier impregnated with component A, age at room temperature for 12 hours, then dry at 120°C for 12 hours, and bake in air at 500°C for 3 hours; the catalyst Before use, it needs to be reduced under hydrogen, the reduction temperature is 200°C, H 2 The pressure is 6MPa, H 2 The flow rate was 160 mL / min, and the catalyst was reduced for 2 hours under this condition to obtain a supported A-B / X catalyst. The composition of catalyst in each embodiment, the kind of starting material of component A, B is shown in Table 1.
[0023] Table 1. The composition of each catalyst ...
Embodiment 9-11
[0028] Catalyst activity evaluation
[0029]The catalyst evaluation of the present invention is carried out in a fixed-bed mobile phase reactor, which is a stainless steel tube with an outer diameter of 6 mm and a length of 360 mm. The experimental process is as follows: 2g of catalyst is loaded into the reaction tube, the catalyst is reduced in situ before the reaction, and the reaction temperature is lowered to the reaction temperature after the reduction, and the H 2 The flow rate is 60mL / min, the flow rate of the organic acid liquid is 0.04mL / min, and the reaction pressure is 6MPa. After 6 hours of reaction, sampling and analysis are carried out.
Embodiment 9
[0031] Activity Evaluation of Acetic Acid Hydrogenation to Ethanol
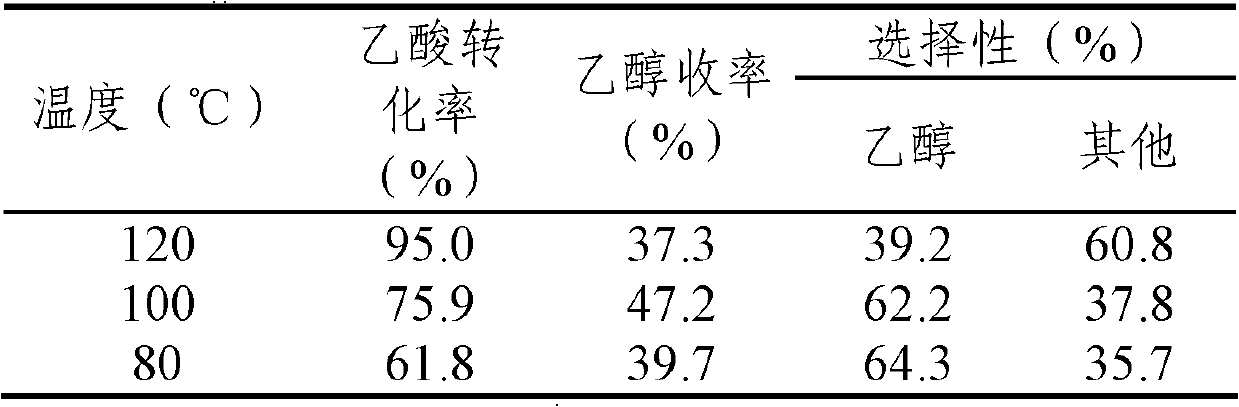
[0032] 1) The influence of reaction temperature on the hydrogenation activity of acetic acid, the activity evaluation results are shown in Table 2.
[0033] Table 2. Effect of reaction temperature on acetic acid hydrogenation activity
[0034]
[0035] Note:
[0036] 4%Ir-MoO x / SiO 2 (Mo / Ir=0.13, x=0~3) Catalyst, 10% acetic acid solution (mass concentration), gas phase products methane, ethane and a small amount of acetaldehyde are represented by "other".
[0037] As can be seen from Table 2, 4% Ir-MoO x / SiO 2 (Mo / Ir=0.13, x=0~3) the catalyst has good low-temperature activity, and when the reaction temperature is 100°C, the yield of ethanol is the highest (47.2%).
[0038] 2) Comparison of the hydrogenation activity of different additives to acetic acid, the activity evaluation results are shown in Table 3.
[0039] Table 3. Effect of different additives on the hydrogenation activity of acetic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com