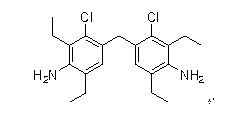
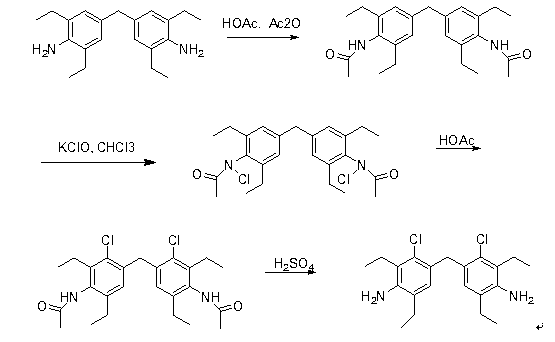
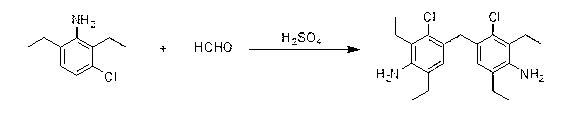Synthetic method of 4,4-methylene bis(3-chloro-2,6-diethyl) aniline
A methylenebis, diethylacetamido technology, applied in the field of polymer material synthesis, can solve the problems of high energy consumption, low production capacity of raw material CDEA, high corrosiveness, etc., achieve high yield and purity, and enhance international competition force effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention will be described in detail below in conjunction with specific embodiments.
[0028] (1) Synthesis of 4,4-methylenebis(2,6-diethylacetamido)benzene
[0029] Dissolve 310 g of 4,4-methylene bis(2,6-diethyl)aniline in 1 kg of acetic acid and slowly add 535 g of acetic anhydride dropwise with constant stirring. After the addition is complete, heat to reflux for 30 mins and vigorously stir, and then The reaction solution was poured into 10 times the volume of cold water and stirred continuously, the precipitated solid was suction filtered and dried to obtain 390 g of the intermediate 4,4-methylenebis(2,6-diethylacetamido)benzene.
[0030] (2) Synthesis of N-chloro-4,4-methylenebis(2,6-diethylacetamido)
[0031] Dissolve 390g of 4,4-methylenebis(2,6-diethylacetamido)benzene in 2L of dichloromethane, add 1500g of sodium hypochlorite solution (available chlorine 10%) twice under stirring and stir overnight at room temperature , the organic phase was sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com