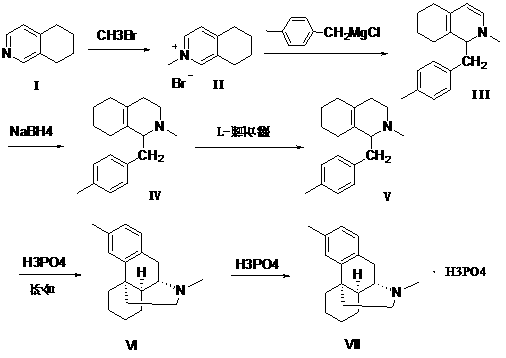
Preparation method of dimemorfan phosphate intermediate
A technology of dimethylphenone phosphate and an intermediate, applied in the field of chemical pharmacy, can solve the problems of unfavorable operation, easy moisture absorption, difficult white flocs and the like, and achieves the effects of ingenious conception, cost reduction and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Under the protection of nitrogen, 30kg (225mol) of 5,6,7,8-tetrahydroisoquinoline and 193kg of tetrahydrofuran were added to the reaction kettle, the temperature was lowered to -15~-10°C, and the reaction solution was directly passed 25.7 kg (270 mol) of methyl bromide was added and reacted for 4 hours. The solvent in the upper layer was extracted, and tetrahydrofuran was added for replacement twice, 49 kg each time. 270 mol of newly prepared p-methylbenzylmagnesium chloride was added, and the reaction was stirred for 4 hours. Add 160kg of methanol to the reaction solution, and then add 7kg of sodium borohydride, and react at 10-30°C for 8 hours. The reaction solution was centrifugally filtered, the filtrate was concentrated to dryness, 160kg of water and 70kg of ethyl acetate were added, the layers were stirred, the water layer was extracted with ethyl acetate 60kg×3, the organic phases were combined, washed with saturated brine 20kg×2, and 30kg of anhydrou...
Embodiment 2
[0033] Example 2: Under the protection of nitrogen, 20.3kg (152.2mol) of 5,6,7,8-tetrahydroisoquinoline and 90kg of tetrahydrofuran were added to the reactor, cooled to -5~0°C, and directly added to the reaction solution 30 kg (273 mol) of methyl bromide was introduced and reacted for 5 hours. The solvent in the upper layer was drawn off, and tetrahydrofuran was added to replace it 3 times. 182 mol of newly prepared p-methylbenzylmagnesium chloride was added, and the reaction was stirred for 3.5 hours. Add 120kg of methanol to the reaction solution, and then add 4.7kg of sodium borohydride, and react at 10-30°C for 8 hours. The reaction solution was centrifugally filtered, the filtrate was concentrated to dryness, 120kg of water and 60kg of ethyl acetate were added, the layers were stirred, the water layer was extracted with 40kg of ethyl acetate x 3, the organic phases were combined, washed with 12kg of saturated brine, and 20kg of anhydrous Dry over sodium sulfate, filter,...
Embodiment 3
[0035] Under the protection of nitrogen, 30.2kg (225mol) of 5,6,7,8-tetrahydroisoquinoline and 120kg of tetrahydrofuran were added to the reaction kettle, and the temperature was lowered to -15~-10°C. 27.8 kg (292 mol) of methyl bromide was introduced and reacted for 5 hours. The solvent in the upper layer was drawn off, and tetrahydrofuran was added for replacement 3 times until TLC showed that there was no raw material point. Add 405 mol of newly prepared p-methylbenzylmagnesium chloride, and stir for 4 hours. Add 162kg of methanol to the reaction solution, then add 7.1kg of sodium borohydride, and react overnight at 10-30°C (about 16 hours). The reaction solution was centrifugally filtered, the filtrate was concentrated to dryness, 120kg of water and 80kg of ethyl acetate were added, the layers were stirred, the water layer was extracted with 40kg of ethyl acetate × 3, the organic phases were combined, washed with 20kg of saturated brine, and 30kg of anhydrous sodium sulfa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com