Synthesis method of Lansoprazole and Lansoprazole synthesized thereby
A synthetic method, the technology of lansoprazole, which is applied in the field of drug preparation, can solve the problems of low purity of lansoprazole and no corresponding improvement of refined products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
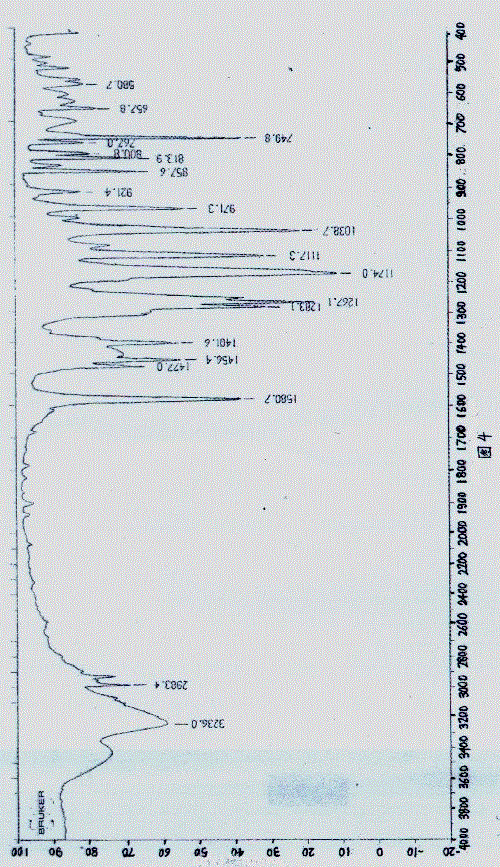
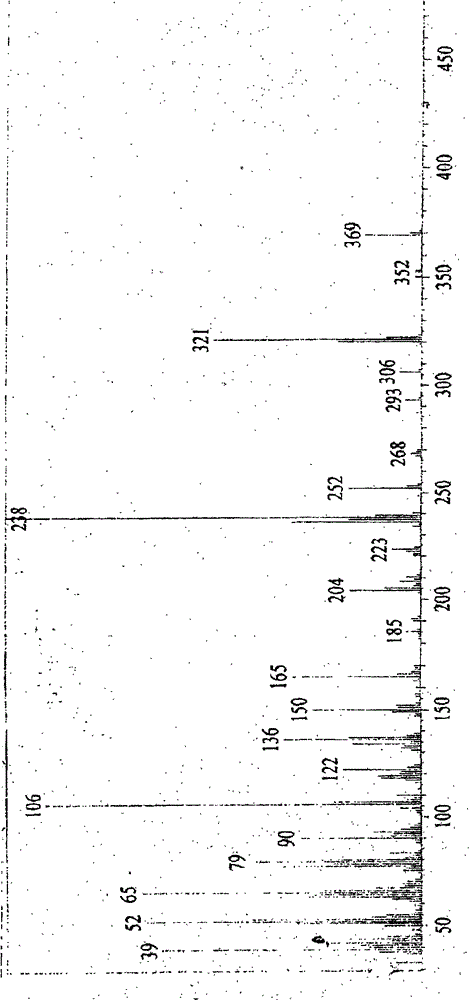
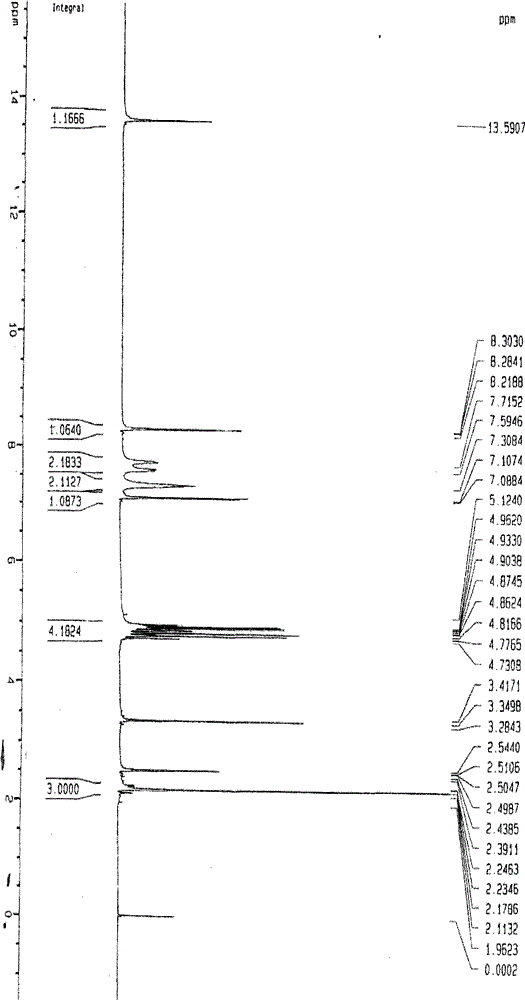
Image
Examples
Embodiment 1
[0035]The synthetic method of 2-[2-[3-methyl-4-(2,2,2-trifluoroethoxy) pyridine] methyl] sulfur-1H-benzimidazole with structural formula (I): in reaction In the bottle, add 1200ml of anhydrous methanol, start stirring, add 105.3g (1.00mol) sodium carbonate, after 10 minutes, add 124.3g (0.45mol) 2-chloromethyl-3-methyl-4-(2,2 , 2-trifluoroethoxy)pyridine hydrochloride and 66.75g (0.44mol) 2-mercapto-benzimidazole, heated to 63°C for reaction after addition, stopped heating and cooled to room temperature 25°C after reaction for 3 hours, filtered to remove Insoluble matter, the filtrate was concentrated under reduced pressure to about 200g, filtered, the filter cake was washed with a small amount of anhydrous methanol, the filtrate was concentrated under reduced pressure until no liquid dripped out, and 200ml of toluene was added to the remaining yellow sticky matter, and stirred at room temperature at 25°C until The solution was turbid, concentrated under reduced pressure to 25...
Embodiment 2
[0057] The synthesis method of the compound with structural formula (1): in the reaction bottle, add absolute ethanol 1000ml, start stirring, add 2-chloromethyl-3-methyl-4-(2.2.2-trifluoroethoxy) 110g (0.40mol) of pyridine hydrochloride, 90g (0.60mol) of 2-mercaptobenzimidazole and 60g (1.50mol) of sodium hydroxide were dissolved in 800ml of ethanol, heated and refluxed for 2 hours, cooled to room temperature 25°C, filtered, and the filtrate Evaporate 2 / 3 of the total amount of ethanol to precipitate a solid, cool to 0°C, filter, wash with water and methanol, and dry under reduced pressure at 45°C to constant weight to obtain 120.5 g of off-white solid, yield: 88.5%, melting point: 147.5 -150.3°C.
[0058] The synthetic method of lansoprazole crude product: in reaction bottle, add in 2000ml ethyl acetate, start to stir, add the compound 106g (0.3mol) that has structural formula (1), be cooled to below 5 ℃, drop m-chlorine A mixed solution of oxybenzoic acid 69.2g (0.4mol) and...
Embodiment 3
[0062] The synthesis method of the compound with structural formula (1): In the reaction flask, add 800ml of anhydrous methanol, slowly add sodium methoxide 86.4g (1.60mol) under stirring, start stirring, add 2-chloromethyl- 110g (0.40mol) of 3-methyl-4-(2,2,2-trifluoroethoxy)pyridine hydrochloride, 60g (0.40mol) of 2-mercaptobenzimidazole, heated at 40°C, stirred, reaction 3 Hour. After the reaction is complete, cool slightly, and filter with suction to obtain the filtrate. The filtrate is evaporated to remove the solvent under reduced pressure, and 600 mL of water is added to the residue. The temperature is raised to 50° C., stirred to dissolve all the solids, and cooled to room temperature. After suction filtration, the filter cake was washed three times with water, and 121.2 g of white solid was dried under reduced pressure at 50°C, yield 85.3%, melting point: 148-151°C.
[0063] The synthetic method of lansoprazole crude product: in reaction bottle, add methanol 1000ml, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com