Novel compound, pharmaceutical compositions thereof as well as preparation methods thereof and use of novel compound and pharmaceutical compositions
A compound and composition technology, applied in the fields of drug combination, steroid compound, sugar derivative preparation, etc., can solve the problems of different effects of anti-tumor activity, weak anti-tumor effect, etc., achieve acute liver injury protection, significantly inhibit tumor Growth effect, effect of strong blood pressure lowering effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
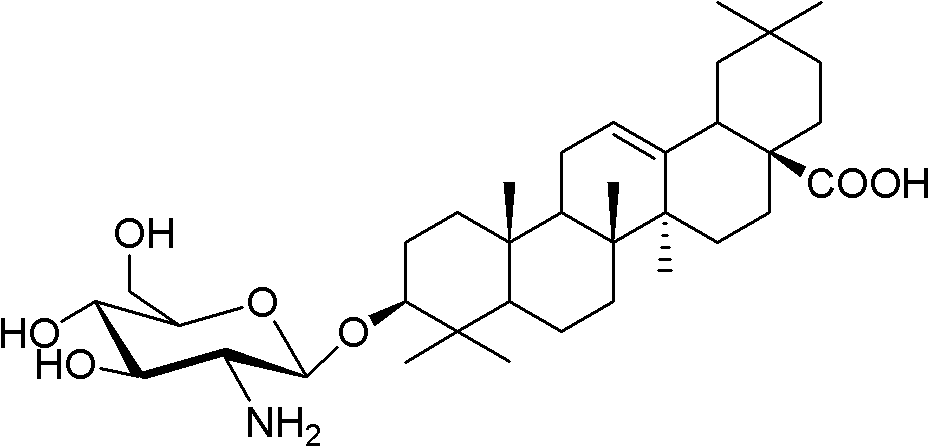
[0045] Embodiment 1: the synthesis of oleanolic acid glucosamine side (I)
[0046] (1) 1,3,4, the synthesis of 6-tetraacetyl-α-D-glucosamine sulfate (formula 2)
[0047]Under cooling in an ice bath, glucosamine hydrochloride (Formula 1, 2Kg, 9.30mol) was slowly added to 10.5L of acetic anhydride. Under rapid stirring, the temperature was controlled not to exceed 30°C, and 930 mL of concentrated sulfuric acid was slowly added dropwise. After the dropwise addition was completed, the mixture was stirred and naturally rose to room temperature. React at 20-25°C until the solution is relatively clear. Immediately cool in an ice bath, and control the temperature not to exceed 30°C. Slowly add absolute ethanol (7L) dropwise to the reaction bottle, and a large amount of white solid immediately appears in the reaction bottle. After the dropwise addition, stir in an ice bath for 30 minutes, filter with suction, and wash the filter cake with ethyl acetate until it has no sour smell. Af...
Embodiment 2
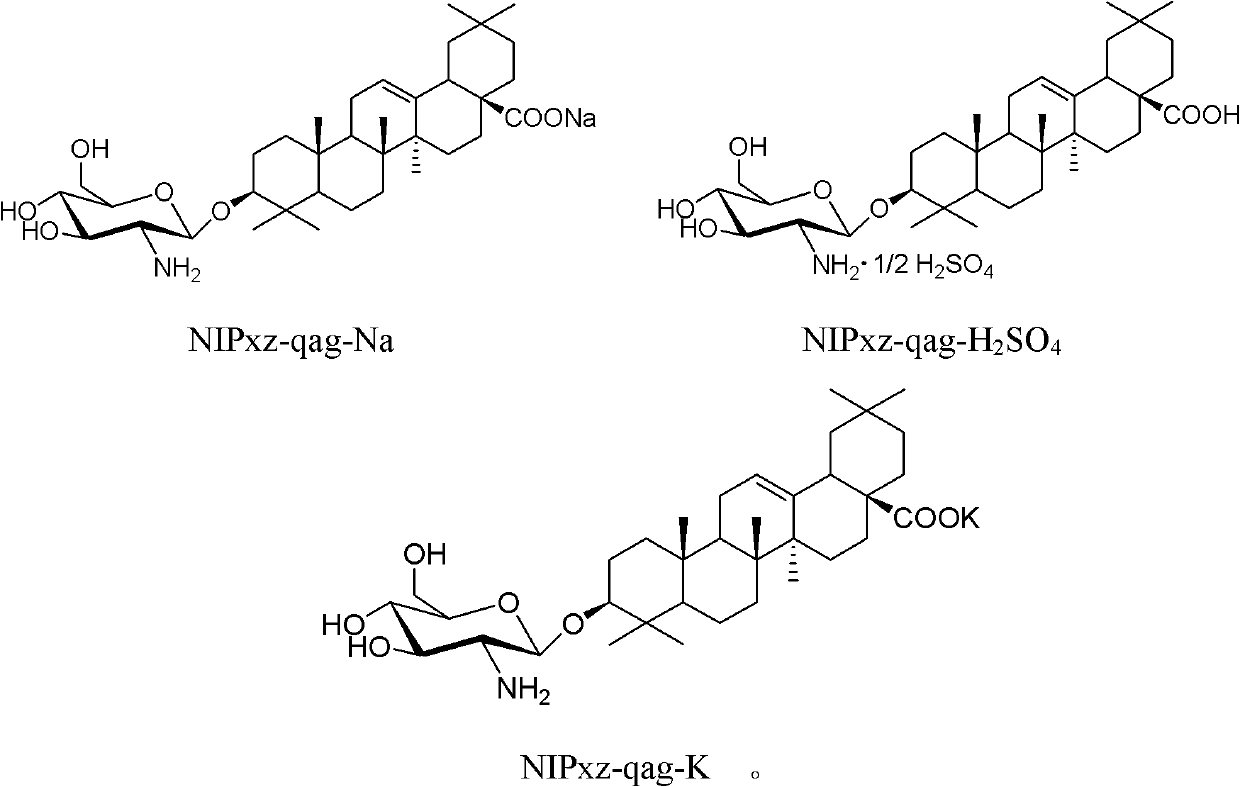
[0064] Embodiment 2: the synthesis of oleanolic acid glucosamine potassium salt (NIPxz-qag-K)
[0065] Under stirring, KOH (1.7 g) was dissolved in 510 mL of ethanol, oleanolic acid glucosamine (Formula I, 15 g) was added, stirred, and heated to reflux for 0.5 h. Cool to room temperature, concentrate under reduced pressure and distill out about 300mL of ethanol. Under stirring, acetonitrile was added dropwise, and a large amount of white solid precipitated out. Suction filtration, wash the filter cake with a small amount of ethanol and acetonitrile, and dry to obtain 13.6 g of oleanolic acid glucosamine potassium salt (NIPxz-qag-K) as a white solid, with a yield of 90%.
[0066] ESI-MS (m / z): 616 [M-K] + . 1 H-NMR (CD 3 OD, 300MHz): δ0.66-1.13(m, 25H), 1.30-1.95(m, 17H), 2.59-2.65(m, 1H), 2.89-2.93(m, 1H), 3.17-3.31(m, 11H ), 3.67(dd, 1H, J=4.6Hz, 11.9Hz), 3.81-3.85(m, 1H), 4.28(d, 1H, J=7.9Hz), 5.20(t, 1H, J=3.4Hz); 13 C-NMR (CD 3 OD, 300MHz): δ6.6, 7.9, 8.9, 10.1, 15....
Embodiment 3
[0067] Embodiment 3: the synthesis of oleanolic acid glucosamine sodium salt (NIPxz-qag-Na)
[0068] Under stirring, dissolve NaOH (0.96g) in 510mL ethanol, add oleanolic acid glucosamine (Formula I, 15g), stir, and heat to reflux for 0.5h. Cool to room temperature, concentrate under reduced pressure and distill out about 300mL of ethanol. Under stirring, acetonitrile was added dropwise, and a large amount of white solid precipitated out. Suction filtration, wash the filter cake with a small amount of ethanol and acetonitrile, and dry to obtain 14.3 g of oleanolic acid glucosamine sodium salt (NIPxz-qag-Na) as a white solid, with a yield of 93%.
[0069] ESI-MS (m / z): 616[M-Na] + . 1 H-NMR (CD 3 OD, 300MHz): δ0.72-1.15(m, 25H), 1.30-1.94(m, 17H), 2.56-2.64(m, 1H), 2.91-2.96(m, 1H), 3.19-3.32(m, 11H ), 3.66 (dd, 1H, J = 4.5Hz, 11.9Hz), 3.82-3.87 (m, 1H), 4.28 (d, 1H, J = 7.9Hz), 5.21 (t, 1H, J = 3.4Hz).
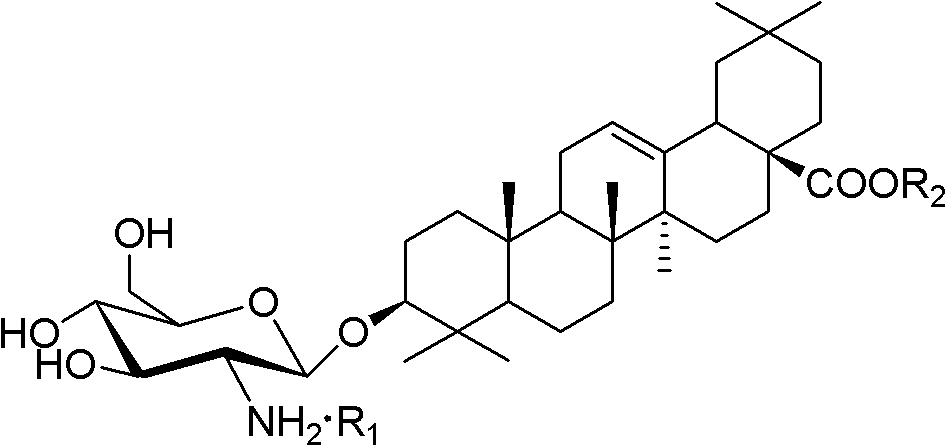
[0070] Referring to the method of Example 2 or 3, oleanolic acid gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com