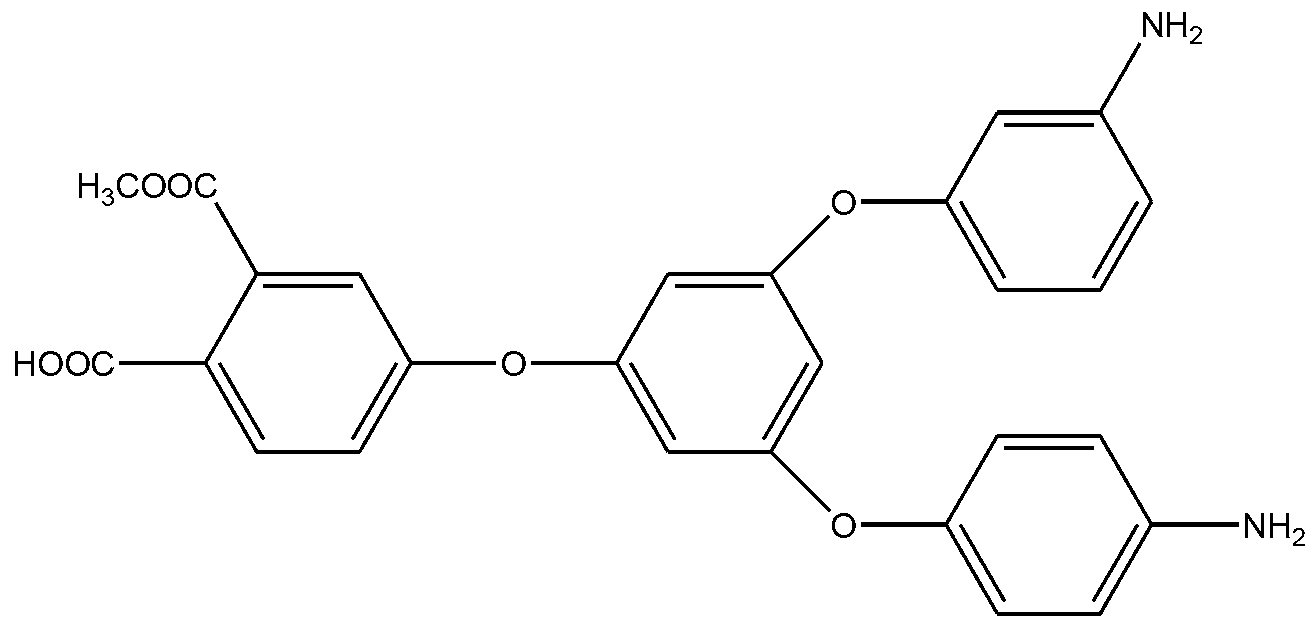
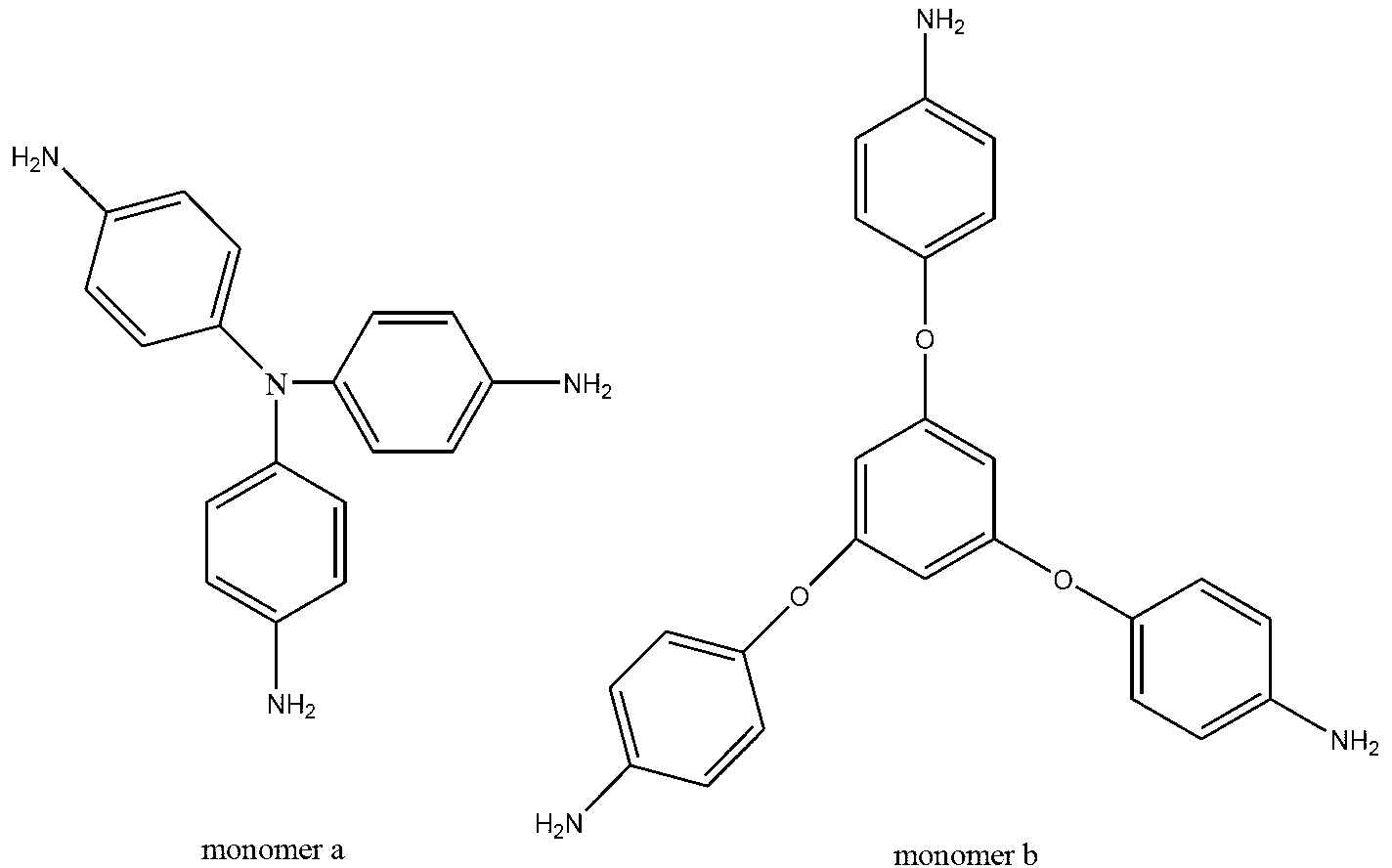
Preparation method of hyperbranched polyimide with adjustable branching degree
A technology of polyimide and degree of branching, applied in the field of synthesis of hyperbranched polymers, can solve the problems of difficult synthesis and easy gel generation, so as to avoid the formation of gel, reduce production cost and increase monomer concentration Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Add 2.5g of 2,4,6-triaminopyrimidine (TAP) and 50g of N,N-dimethylacetamide (DMAc) into a completely dry 150mL straight four-necked flask equipped with a water separator, At 25°C, with mechanical stirring and N 2 Under protection, 8.88 g of hexafluorodianhydride (6FDA) monomer was added after stirring for 5 minutes, and the bottle wall was washed with 14.5 g of DMAc. The stirring reaction was continued for 48h.
[0023] (2) The temperature of the above solution was raised to 160° C., 20 g of xylene was added, reacted for 6 hours, and the water generated during the imidization process was removed by azeotroping of xylene and water.
[0024] (3) After cooling to room temperature, the above solution was poured into ethanol, washed with ethanol, filtered three times, and vacuum-dried at 150°C for 6 hours to obtain a hyperbranched polyimide with a branching degree of 34%.
Embodiment 2
[0026] (1) Add 2.0833g TAP and 50g DMAc to a completely dry 150mL straight four-neck flask equipped with a water separator, and add 2.0833g TAP and 50g DMAc at 25°C, mechanical stirring and N 2 Under protection, 8.88g of 6FDA monomer was added after stirring for 5 minutes, and the bottle wall was washed with 12.1g of DMAc. The stirring reaction was continued for 48h.
[0027] (2) The temperature of the above solution was raised to 160° C., 20 g of xylene was added, reacted for 6 hours, and the water generated during the imidization process was removed by azeotroping of xylene and water.
[0028] (3) After cooling to room temperature, the above solution was poured into ethanol, washed with ethanol, filtered three times, and vacuum-dried at 150°C for 6 hours to obtain a hyperbranched polyimide with a branching degree of 42%.
Embodiment 3
[0030] (1) Add 1.7857g TAP and 45g DMAc to a completely dry 150mL straight four-neck flask equipped with a water trap, and add 1.7857g TAP and 45g DMAc at 25°C, mechanical stirring and N 2 Under protection, 8.88g of 6FDA monomer was added after stirring for 5 minutes, and the bottle wall was washed with 15.4g of DMAc. The stirring reaction was continued for 48h.
[0031] (2) The temperature of the above solution was raised to 160° C., 20 g of xylene was added, reacted for 6 hours, and the water generated during the imidization process was removed by azeotroping of xylene and water.
[0032] (3) After cooling to room temperature, the above solution was poured into ethanol, washed with ethanol, filtered three times, and vacuum-dried at 150°C for 6 hours to obtain a hyperbranched polyimide with a branching degree of 53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com