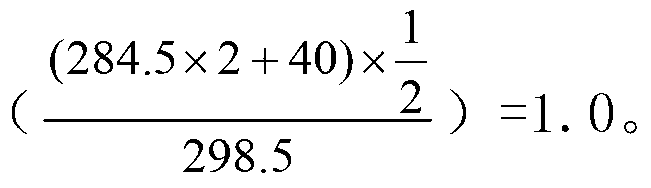Method for measuring calcium stearate in drug
A technology of calcium stearate and methyl stearate, applied in the field of analytical chemistry, can solve problems such as inability to accurately quantify calcium stearate, and achieve the effects of fast determination method and low detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the mensuration of calcium stearate in sodium chloride injection
[0029] 1. Sample preparation:
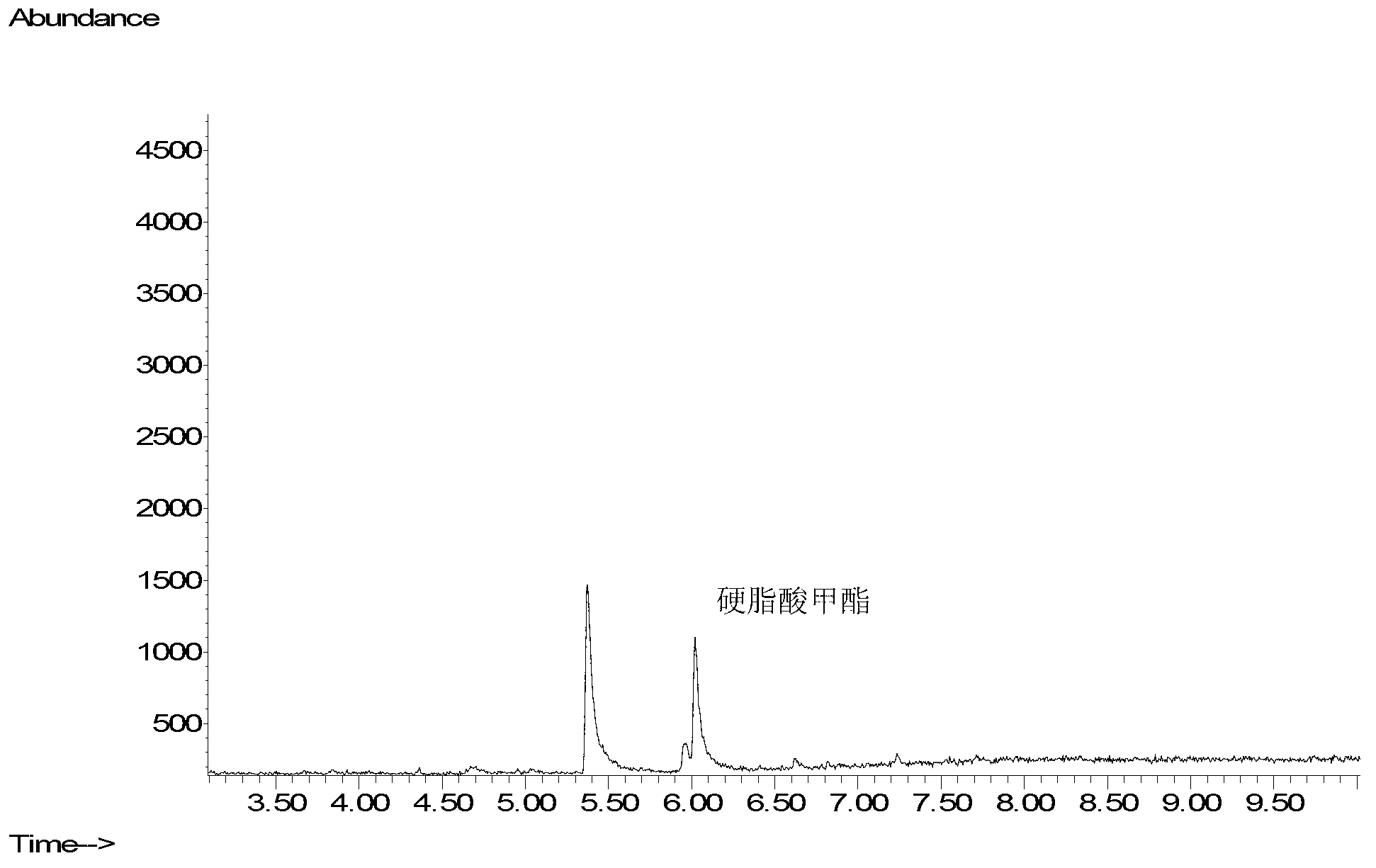
[0030] Take 4 mL of sodium chloride injection, add 400 mL of hydrochloric acid, mix well, and ultrasonically acidify in a water bath at 60 ° C for 1 hour. After the sample is cooled, transfer the sample solution to a 125 mL separating funnel, add 20 mL of water and mix well, divide it into 3 times with 60 mL of n-hexane For extraction, the n-hexane extract was dehydrated with anhydrous sodium sulfate, concentrated to less than 2mL with a rotary evaporator at 40°C, transferred the solution to a 10mL glass centrifuge tube, and blown dry with nitrogen.
[0031] Add 1mL of methanol to dissolve the residue, add 100uL of concentrated sulfuric acid and mix well, heat in a water bath at 30°C for 20 minutes, add 2mL of water and 1mL of n-hexane, vortex mix for 1 minute, let stand to separate layers, and take the n-hexane layer for GC / MS analysis. Measure the amount o...
Embodiment 2
[0054] Embodiment 2: the mensuration of calcium stearate in the antibiotic cefminox sodium for injection
[0055] 1. Sample preparation:
[0056] Weigh 4.0g of uniform drug powder, add 40ml of water to ultrasonically dissolve the sample, pipette 2mL of the sample solution into a 40mL glass bottle, add 400% hydrochloric acid, mix well, ultrasonically acidify in a water bath at 60°C for 1 hour, and transfer the sample solution to 125mL after the sample is cooled In the separatory funnel, add 20mL water and mix well, extract 60mL n-hexane for 3 times, dehydrate the n-hexane extract with anhydrous sodium sulfate, concentrate to less than 2mL with a rotary evaporator at 40°C, transfer the solution to a 10mL glass centrifuge tube, blow with nitrogen Dry.
[0057]Add 1mL of methanol to dissolve the residue, add 100uL of concentrated sulfuric acid and mix well, heat in a water bath at 30°C for 20 minutes, add 2mL of water and 1mL of n-hexane, vortex mix for 1 minute, let stand to sep...
Embodiment 3
[0081] Embodiment 3: the mensuration of calcium stearate in the antibiotic cefoxitin sodium for injection
[0082] 1. Sample preparation:
[0083] Weigh 4.0g of uniform drug powder, add 40ml of water to ultrasonically dissolve the sample, pipette 2mL of the sample solution into a 40mL glass bottle, add 400% hydrochloric acid, mix well, ultrasonically acidify in a water bath at 60°C for 1 hour, and transfer the sample solution to 125mL after the sample is cooled In the separatory funnel, add 20mL water and mix well, extract 60mL n-hexane for 3 times, dehydrate the n-hexane extract with anhydrous sodium sulfate, concentrate to less than 2mL with a rotary evaporator at 40°C, transfer the solution to a 10mL glass centrifuge tube, blow with nitrogen Dry.
[0084] Add 1mL of methanol to dissolve the residue, add 100uL of concentrated sulfuric acid and mix well, heat in a water bath at 30°C for 20 minutes, add 2mL of water and 1mL of n-hexane, vortex mix for 1 minute, let stand to s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com