Moxifloxacin hydrochloride-containing pharmaceutical composition and preparation method thereof
A technology of moxifloxacin hydrochloride and its composition, which is applied in the field of pharmaceutical composition containing moxifloxacin hydrochloride and its preparation, can solve the problem of flying dust, not being able to meet the requirement that the bioavailability of tablets is not limited by dissolution, and unfavorable for laborers Protection and other issues to achieve the effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
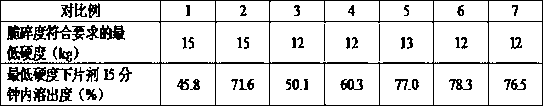
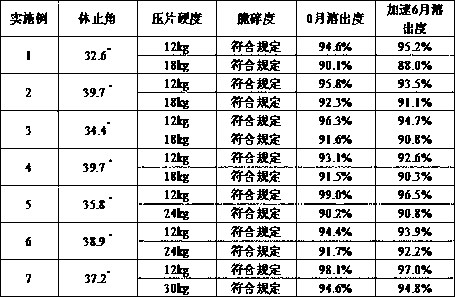
Image
Examples
Embodiment 1
[0101] Material Weight (mg) Percentage
[0102] Moxifloxacin hydrochloride 436.4 43.64%
[0103] Microcrystalline Cellulose 10.0 1.00%
[0104] Mannitol 403.6 40.36%
[0105] Hypromellose 100.0 10.00%
[0106] Sodium Lauryl Sulfate 50.0 5.00%
[0107] ______________________________________________________________
[0108] Total 1000 100.00%
[0109] Mix moxifloxacin hydrochloride, mannitol, microcrystalline cellulose, and hydroxypropyl cellulose, moisten it with water, granulate it, dry it, granulate it, mix it with sodium lauryl sulfate, and make it with a hardness of 12kg and 18kg respectively. into tablets.
Embodiment 2
[0111] Material Weight (mg) Percentage
[0112] Moxifloxacin hydrochloride 436.4 43.64%
[0113] Microcrystalline cellulose 461.6 46.16%
[0114] Mannitol 90.0 9.00%
[0115] Croscarmellose Sodium 10.0 1.00%
[0116] Magnesium Stearate 2.0 0.20%
[0117] ______________________________________________________________
[0118] Total 1000 100.00%
[0119] Mix moxifloxacin hydrochloride, mannitol, and microcrystalline cellulose, moisten with water, granulate, dry, granulate, then mix with croscarmellose sodium, magnesium stearate, and use hardness 12kg and 18kg Tablets are made separately.
Embodiment 3
[0121] Material Weight (mg) Percentage
[0122] Moxifloxacin hydrochloride 436.4 54.55%
[0123] Microcrystalline cellulose 80.0 10.00%
[0124] Mannitol 179.6 22.45%
[0125] Croscarmellose Sodium 80.0 10.00%
[0126] Magnesium Stearate 24.0 3.00%
[0127] ______________________________________________________________
[0128] Total 800 100.00%
[0129] Mix moxifloxacin hydrochloride, mannitol, microcrystalline cellulose, and croscarmellose sodium, moisten it with water, granulate it, dry it, granulate it, and mix it with magnesium stearate evenly. Tablets are made separately.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com