Small-volume moxifloxacin hydrochloride injection and preparation method thereof
A technology of moxifloxacin hydrochloride and moxifloxacin, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery, can solve the problems of inability to use sodium chloride, unsuitable for clinical application, and large floor space, etc. Achieve the effect of good clarity, high sterilization guarantee value and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of small volume injection of moxifloxacin hydrochloride
[0043] Table 4 Moxifloxacin Hydrochloride Small Volume Injection Prescription
[0044] Moxifloxacin hydrochloride (calculated as moxifloxacin) 200g 0.5mol / L sodium hydroxide solution Appropriate amount Water for Injection Appropriate amount Full amount 10000ml
[0045] According to the above prescription, three batches of samples were continuously prepared, each batch was 2000ml, numbered 20110901, 20110902, 20110903, and the preparation method was as follows;
[0046] Preparation Process:
[0047] (1) Weigh the prescribed amount of moxifloxacin hydrochloride 200g and add it to 90% of the total volume of water for injection, stir at 60-80°C for 15 minutes to fully dissolve the main drug;
[0048] (2) Supplement an appropriate amount of water for injection to make up the volume to full volume;
[0049] (3) Then add an appropriate amount of 0.5mol / L sodi...
Embodiment 2
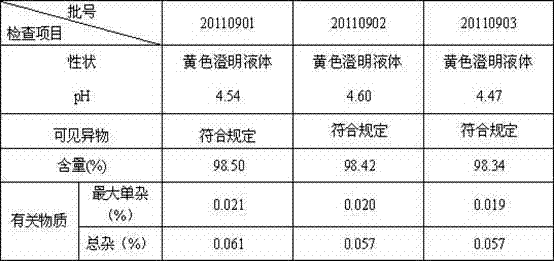
[0061] Example 2 Three batches of samples in Example 1 are detected
[0062] The finished product of each batch in the embodiment is checked, and sample is carried out the mensuration of pH, content, related substance, and observes its outward appearance, and the results are shown in the following table:
[0063] Table 8 Test results of three batches of small test samples
[0064]
[0065] The results show that all the indicators of the moxifloxacin hydrochloride injection of the present invention meet the quality standard requirements.
Embodiment 3
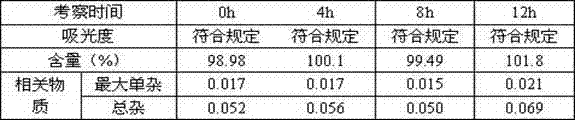
[0066] Example 3 investigates the solution stability prepared under normal light conditions
[0067] Since the color of the moxifloxacin hydrochloride raw material is darkened under the light conditions of the influencing factors, and the related substances and contents have no obvious changes, so the preparation of this product is investigated under normal light conditions, and the solution is placed under normal light conditions for 12 hours, measured 0 , 4, 8, 12h absorbance, content and related substances, the results are as follows:
[0068] The solution stability result prepared under the normal light condition of table 9
[0069]
[0070] It can be seen from the experimental results that the color, content and related substances of the moxifloxacin hydrochloride solution prepared under normal light conditions meet the requirements, and compared with 0h after being placed under normal light conditions for 12 hours, the absorbance, content and related substances hav...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com