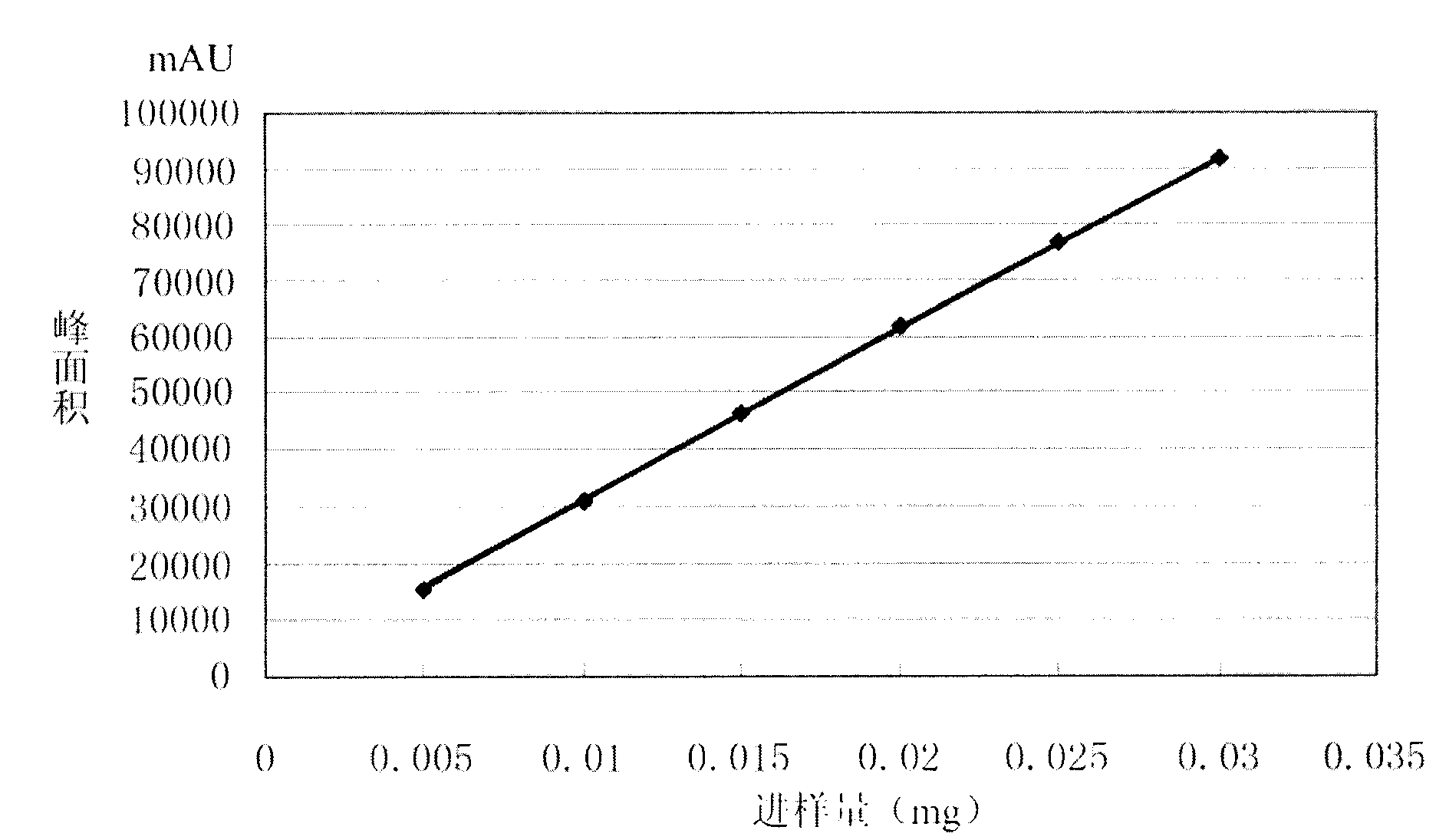Detection method of moxifloxacin (R, R) isomer and application thereof
A detection method and mobile phase technology, applied in the field of detection of -isomers, can solve problems affecting the calculation of effective substance content in preparations, achieve high sensitivity and specificity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Instruments and testing conditions
[0033] The Agilent 1260 high-performance liquid chromatograph produced by Agilent Corporation of the United States, the ZORBAX Eclipse Plus C18 chromatographic column (column specification: 4.6 × 250mm, 5 μm) produced by Agilent Technology Co., Ltd.; detection wavelength: 293nm; mobile phase: add L- Solvent for leucine and copper sulfate, the solvent is composed of water and acetonitrile, the ratio is to be selected, the concentration of L-leucine and copper sulfate is 0.05mol / L; flow rate: 1ml / min; column temperature: 35 ℃; injection volume: 20 μl.
[0034] 2) Experimental steps
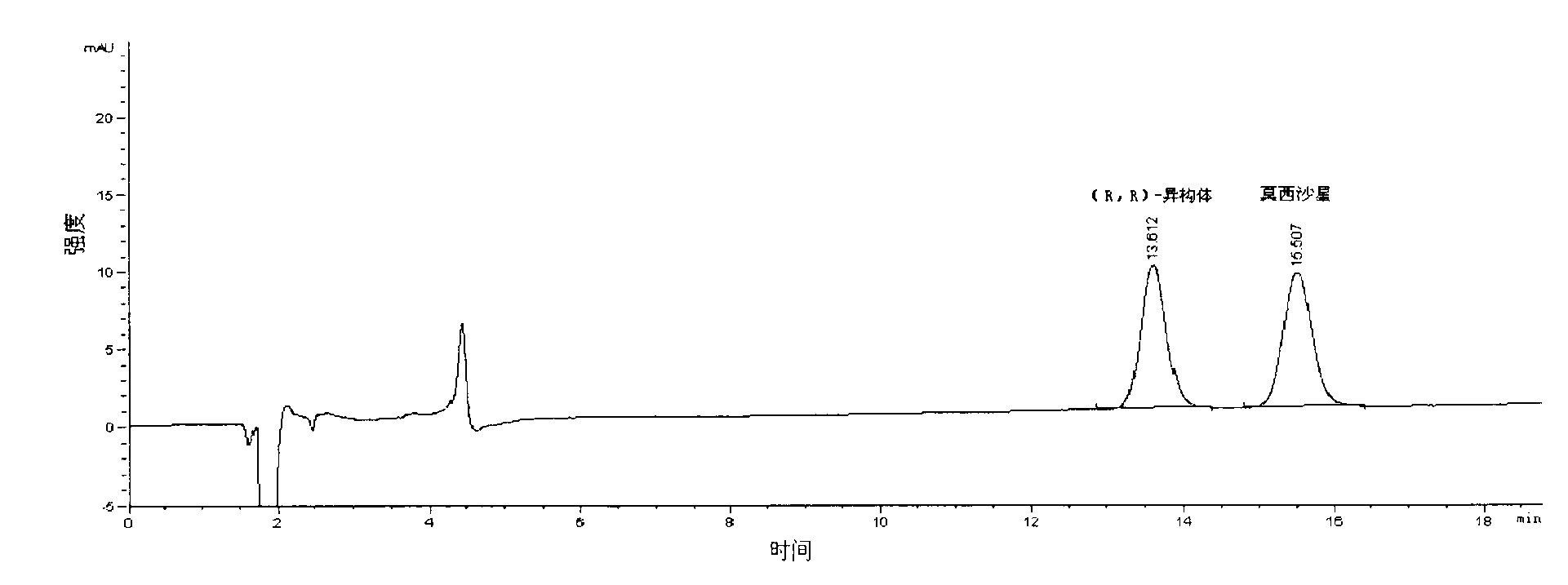
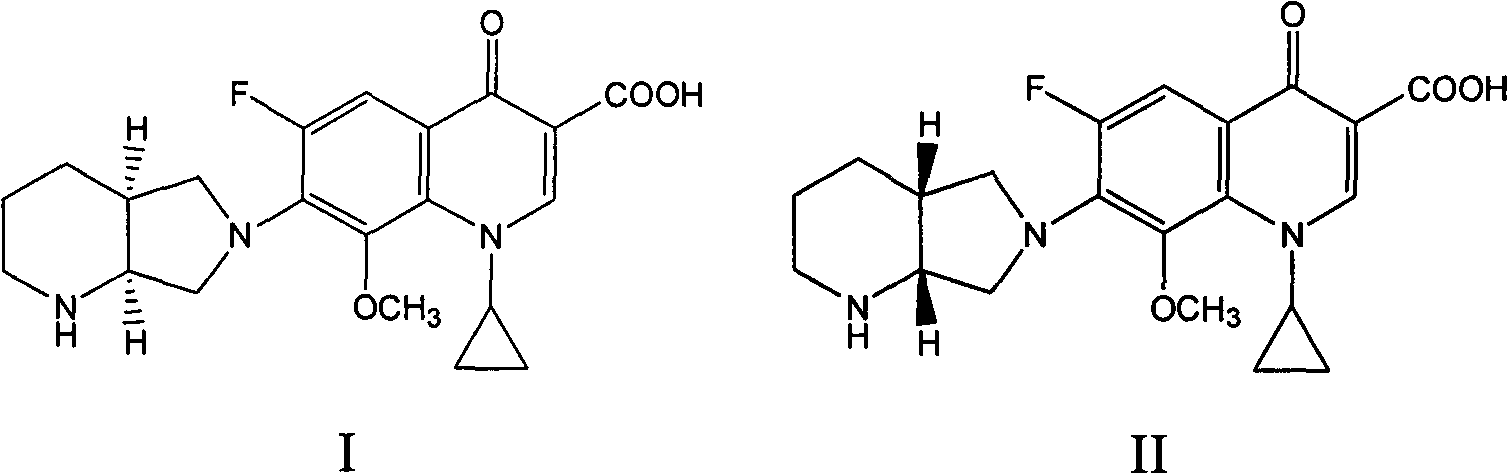
[0035] Take an appropriate amount of the mixture composed of moxifloxacin and moxifloxacin (R, R)-isomers, add mobile phase to dissolve, and prepare a test solution with a concentration of 0.005mg / ml, and take moxifloxacin and each optical isomer The standard product of the body was configured as a standard solution as a control.
[0036] Weigh the t...
Embodiment 2
[0042] Take water as solvent, take moxifloxacin sample, prepare the solution that concentration is 1mg / ml as need testing solution, then carry out following experiment respectively, to test the specificity of the inventive method:
[0043] 1) Take 10ml of the test solution in a stoppered test tube, add 1ml of hydrochloric acid solution with a concentration of 0.1mol / L, heat it in a water bath at 90°C for 1 hour, let it cool naturally, and use a concentration of 0.1mol / L sodium hydroxide The solution is adjusted to neutral;
[0044] 2) Take 10ml of the test solution in a stoppered test tube, add 1ml of sodium hydroxide solution with a concentration of 0.1mol / L, heat it in a water bath at 90°C for 1 hour, let it cool naturally, and use 0.1mol / L sodium hydroxide solution The hydrochloric acid solution was adjusted to neutrality;
[0045] 3) Take 10ml of the test solution and place it in a stoppered test tube, heat it in a water bath at 90°C for 1 hour, leave it to cool naturally...
Embodiment 3
[0051]1) Instruments and testing conditions: Agilent 1260 high performance liquid chromatography produced by Agilent Corporation of the United States, a chromatographic column ZORBAX Eclipse Plus C18 produced by Agilent Technologies Co., Ltd. (column specification: 4.6×250mm, 5μm); detection wavelength: 293nm Mobile phase: add the solvent of chiral reagent L-leucine and copper sulfate, solvent is made up of water and acetonitrile, and volume ratio is 80: 20, and the concentration of L-leucine and copper sulfate is 0.05mol / L; : 1ml / min; Column temperature: 35°C.
[0052] 2) Preparation of the solution:
[0053] Accurately weigh 10 mg of moxifloxacin sample and add it to a 10 ml measuring bottle, dissolve it ultrasonically with pure water and dilute to the mark as the sample solution.
[0054] In addition, accurately weigh 10 mg of moxifloxacin standard substance and add it to a 10 ml volumetric flask, ultrasonically dissolve it with pure water and dilute to the mark, accuratel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com