Moxifloxacin hydrochloride external preparation and preparation method thereof
A technology of moxifloxacin hydrochloride and ointment, which is applied in the field of external preparations of moxifloxacin hydrochloride, can solve the problems of insufficient function and low penetration concentration, and achieve the effects of promoting wound healing, rapid penetration and absorption, and promoting absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
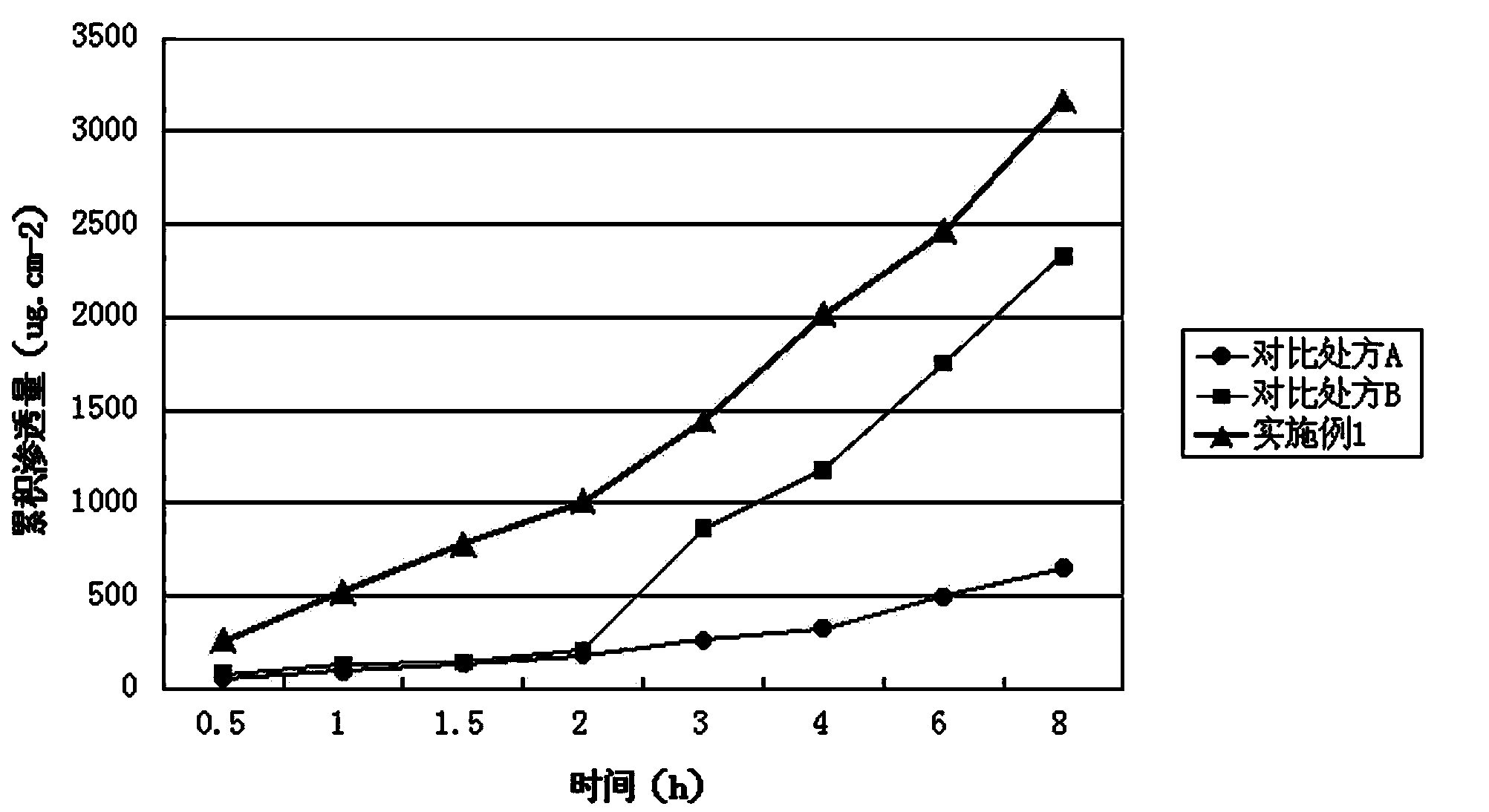
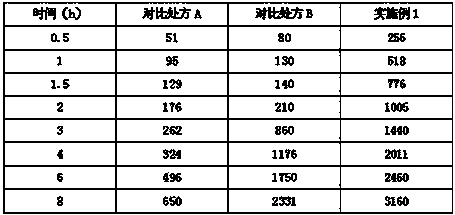
Image
Examples
Embodiment 1
[0041] The prescription is as follows:
[0042] Moxifloxacin Hydrochloride 0.5%
[0043] Stearic Acid 30%
[0044] Glyceryl Monostearate 15%
[0045] White Vaseline 10%
[0046] Lanolin 12%
[0047] Glycerin 8%
[0048] Ethylparaben 0.06%
[0049] Edetate Disodium 0.05%
[0050] Triethanolamine 4%
[0051] N-Trimethyl Chitosan 3%
[0052] Menthol 2%
[0053] Isopropyl myristate 1%
[0054] Purified water balance.
[0055] Preparation method: The following materials are fed according to the prescription quantity
[0056] 1) Heat stearic acid, glyceryl monostearate, lanolin, and white petrolatum to 75-80°C and mix well to obtain the oil phase;
[0057] 2) First put N-trimethyl chitosan into 75% of the prescription amount of purified water, stir until it is completely dissolved, then add menthol, after stirring evenly, add disodium edetate, ethylparaben, and triethanolamine in sequence , heated to 75-80°C and stirred evenly to obtain an aqueous phase;
[0058] 3) Ad...
Embodiment 2
[0062] The prescription is as follows:
[0063] Moxifloxacin Hydrochloride 0.8%
[0064] Stearic Acid 20%
[0065] Glyceryl Monostearate 10%
[0066] White Vaseline 8%
[0067] Lanolin 10%
[0068] Glycerin 6%
[0069] Chlorobutanol 0.04%
[0070] Edetate Disodium 0.05%
[0071] Triethanolamine 3%
[0072] N-Trimethyl Chitosan 3%
[0073] Menthol 2%
[0074] Isopropyl myristate 1%
[0075] Purified water balance.
[0076] Preparation method: The following materials are fed according to the prescription quantity
[0077] 1) Heat stearic acid, glyceryl monostearate, lanolin, and white petrolatum to 75-80°C and mix well to obtain the oil phase;
[0078] 2) First put N-trimethyl chitosan into 75% of the prescription amount of purified water, stir until completely dissolved, then add menthol, stir well, then add disodium edetate, chlorobutanol, triethanolamine in turn , heated to 75-80°C and stirred evenly to obtain an aqueous phase;
[0079] 3) Add the water phase to...
Embodiment 3
[0083] The prescription is as follows:
[0084] Moxifloxacin Hydrochloride 0.5%
[0085] Butyl flufenamate 0.1%
[0086] Stearic Acid 20%
[0087] Glyceryl Monostearate 10%
[0088] White Vaseline 8%
[0089] Lanolin 10%
[0090] Glycerin 6%
[0091] Ethylparaben 0.04%
[0092] Edetate Disodium 0.05%
[0093] Triethanolamine 3%
[0094] N-Trimethyl Chitosan 3%
[0095] Menthol 2%
[0096] Isopropyl myristate 1%
[0097] Purified water balance.
[0098] Preparation method: The following materials are fed according to the prescription quantity
[0099] 1) Heat stearic acid, glyceryl monostearate, lanolin, and white petrolatum to 75-80°C and mix well to obtain the oil phase;
[0100] 2) First put N-trimethyl chitosan into 75% of the prescription amount of purified water, stir until it is completely dissolved, then add menthol, after stirring evenly, add disodium edetate, ethylparaben, and triethanolamine in sequence , heated to 75-80°C and stirred evenly to obtain a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com