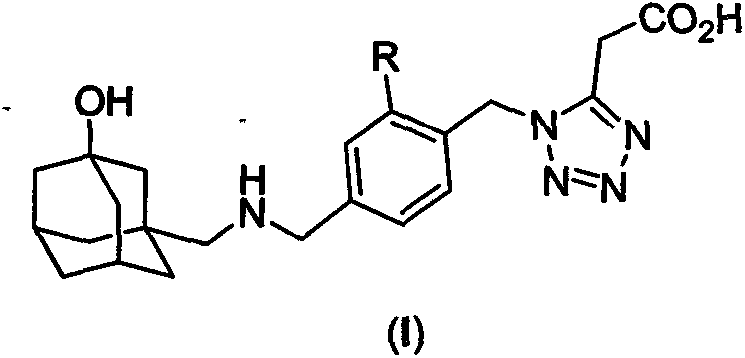
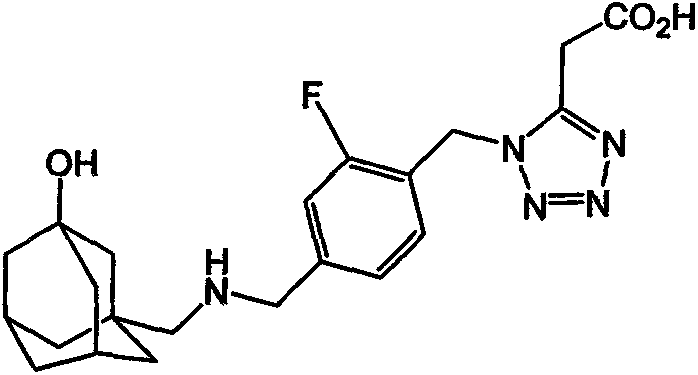
Anti-diabetic compound, as well as preparation method and application thereof
A technology for diabetes drugs and compounds, applied in the fields of active ingredients of heterocyclic compounds, metabolic diseases, organic chemistry, etc., which can solve problems such as weight gain and obvious side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] The starting materials for the reaction were commercially available.
[0024] 1.81g (10mmol) compound A and 1.54g (10mmol) compound B-1 were dissolved in 20mL EtOH, reacted at room temperature for 3 hours and then added 1.89g (30mmol) NaBH 3 CN, then continued to stir overnight. The reaction mixture was poured into 100 mL of ice water, stirred, adjusted to pH = 6 with concentrated hydrochloric acid, extracted with 50 mL×3 dichloromethane, combined the extract phases, washed once with brine, dried over anhydrous sodium sulfate, and distilled off the solvent in a rotary evaporator to obtain A residue, then purified by column chromatography to obtain the pure product of C-1, ESI-MS, m / z=337 ([M+NH 4 ] + ).
[0025] Dissolve 2.23g (7mmol) of compound C-1 in 10mL of dry toluene, stir slowly under cooling in an ice-water bath, and slowly add 2.71g (10mmol) of PBr 3 Dissolve in 2 mL of dry dichloromethane solution. After the dropwise addition, the reaction mixt...
Embodiment 2-4
[0028] According to the method of Example 1, the compounds of general formula I shown in the table below were prepared.
[0029]
Embodiment 5
[0031] The sample was formulated with 1% sodium carboxymethylcellulose into a suspension at a concentration of 5 mg / mL, and the administration volume was 0.4 mL / 20 g of body weight, equivalent to a dose of 100 mg / kg.
[0032] Healthy ICR mice, half male and half male, weighing 20-24g, meet the first-class standard. Animals were fasted for 16 hours, given the compound to be tested by intraperitoneal injection of 2g / kg glucose saline solution for 15 minutes after intraperitoneal injection, and were regularly taken from the retrobulbar venous plexus of mice at 0.5h, 1h, 1.5h and 2h after modeling. Blood was centrifuged to separate serum, and the serum glucose content at each time point was determined by glucose oxidase method. The blank mice were given neither glucose nor the test compound, and the model mice were only given glucose without the test compound.
[0033]
[0034] It can be seen from the data in the above table that the compound of the present invention can signi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com