Magnetic bead separation method of escherichia coli O157
A technology for separation of Escherichia coli and magnetic beads, applied in the biological field, can solve the problems of separation failure, poor monodispersity of micro-magnetic beads, and large concentration of bacteria, and achieve the effect of increasing the chance of contact, shortening the separation time, and improving the capture efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
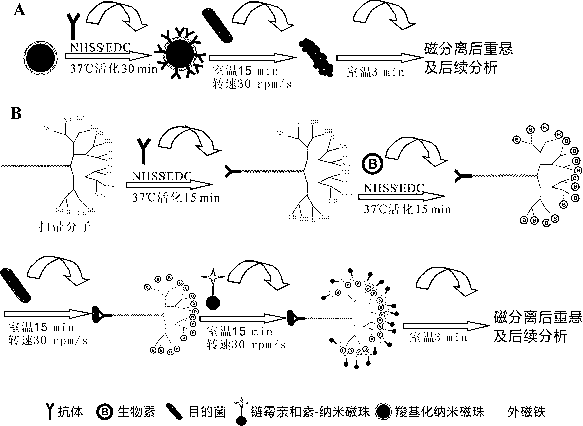
[0036] 1. The broom molecule-antibody complex is prepared according to the following steps:
[0037] (1) Dissolve 1 mg of aminated broom molecule in 2 mL of 0.02 M, pH 6.5 phosphate buffer PBS, add 0.6 mg of N-hydroxysuccinimide NHSS, 0.4 mg of ethyl 3-(3-dimethylamino ) carbodiimide hydrochloride EDC, placed on a mixer at room temperature and stirred, activated for 15 min;
[0038] (2) Take 11.5 mg E. coli O157 The specific antibody was added to the above reaction solution, placed on a mixer at room temperature and stirred for 30 min;
[0039] (3) The above solution was spin-dried under reduced pressure, dissolved in deionized water, and dialyzed in PBS and deionized water for 1 day; after the dialysis, the obtained solution was freeze-dried.
[0040] 2. The long-chain biotin-broom molecule-antibody complex is prepared according to the following steps:
[0041] (1) Dissolve 10 mg long-chain biotin, 3.6 mg NHSS, and 2.4 mg EDC in 2 mL 0.02 M pH 6.5 PBS buffer;
[0042] (2...
Embodiment 2
[0046] Example 2 Enrichment effect experiment
[0047] (1) Take 1 mL of concentration as 10 4 cfu / mL E. coli O157 Centrifuge at 12,000 rpm for 5 min in a 1.5 mL sterile centrifuge tube, discard the supernatant, and resuspend with an equal volume of sterile PBS solution.
[0048] (2) Enrichment and capture: respectively set the technical solution group of the present invention ( E. coli O157 Antibody and long-chain biotin co-modified broom group), E. coli O157 Specific antibody-modified nano-magnetic bead set, E. coli O157 Specific antibody-modified micron magnetic bead group enriches target bacteria.
[0049] (3) After magnetic separation, pour the supernatant into a sterile centrifuge tube, and capture the E. coli O157 The immunomagnetic beads were washed twice with PBST, mixed well, and the immunomagnetic bead complex was resuspended with 1 mL sterile PBS solution.
[0050] (4) Capture rate calculation: After gradient dilution of the enriched target bacteria resu...
Embodiment 3
[0063] Example 3 Enrichment capture experiment
[0064] Conventional magnetic stand separation time is 30min, and all the other are with embodiment 2.
[0065] The catch rate of each group is as follows:
[0066] E. coli O157 Specific antibody-modified micron magnetic beads Obtaining rate E. coli O157 Capture efficiency of specific antibody-modified nanomagnetic bead sets E. coli O157 Capture efficiency of broom groups co-modified with antibodies and long-chain biotin 58.7% 39.1% 92.1%
[0067] Experimental result shows, separates 3min among the comparative example 2, when separation time reaches 30min, the capture efficiency of three groups has all been improved, especially E. coli O157 The capture efficiency of the specific antibody-modified nano-magnetic bead group is the most obvious, which shows that the capture efficiency of the nano-magnetic bead group can be greatly improved by extending the time, but it is still lower than the short-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com