Fullerene derivative micrometer flower and preparation method thereof
A technology of fullerene derivatives and micron flowers, applied in organic chemistry and other fields, can solve the problems of large powder size and inability to obtain products, and achieve the effects of increased specific surface area, cheap raw materials, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The concrete steps of preparation are:
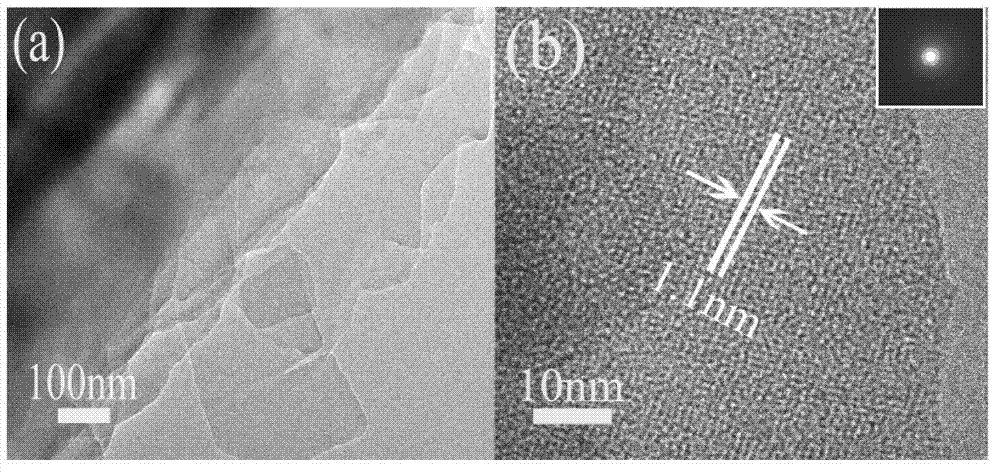
[0025] Concentration is the N-methyl-2-[4-dimethylamino] phenyl-3,4-fullerenyl pyrrolidine carbon tetrachloride solution that concentration is 0.75g / L and concentration is 7mmol / L ten After mixing the hexaalkyltrimethylammonium bromide isopropanol solution, stir at 10°C for 30 minutes to obtain a mixed solution; wherein, N-methyl-2-[4-dimethylamino]phenyl-3 , The volume ratio of 4-fullerenylpyrrolidine carbon tetrachloride solution to cetyltrimethylammonium bromide isopropanol solution is 1:1. After the mixed solution is alternately subjected to solid-liquid separation and washing for 3 times, it is placed at room temperature and dried for 10 minutes; wherein, the solid-liquid separation process is centrifugation, and the rotating speed during centrifugation is 4000r / min, and the time is 12min, the washing process is to use ethanol to clean the solid matter obtained after centrifugation, and obtain a product similar to figure 1...
Embodiment 2
[0027] The concrete steps of preparation are:
[0028] Concentration is the N-methyl-2-[4-dimethylamino] phenyl-3,4-fullerenyl pyrrolidine carbon tetrachloride solution that concentration is 1.06g / L and the decathyl chloride solution that concentration is 6mmol / L After mixing the hexaalkyltrimethylammonium bromide isopropanol solutions, stir at 17°C for 28 minutes to obtain a mixed solution; wherein, N-methyl-2-[4-dimethylamino]phenyl-3 , The volume ratio of 4-fullerenylpyrrolidine carbon tetrachloride solution to cetyltrimethylammonium bromide isopropanol solution is 1:2.3. After the solid-liquid separation and washing treatment were carried out alternately to the mixed solution for 3 times, it was placed at normal temperature and dried for 10 minutes; wherein, the solid-liquid separation process was centrifugation, and the rotating speed during centrifugation was 4500r / min, and the time was 11min, the washing process is to use ethanol to clean the solid matter obtained afte...
Embodiment 3
[0030] The concrete steps of preparation are:
[0031] Concentration is the N-methyl-2-[4-dimethylamino] phenyl-3,4-fullerenyl pyrrolidine carbon tetrachloride solution of 1.37g / L and the decathyl chloride solution of 5mmol / L After mixing the hexaalkyltrimethylammonium bromide isopropanol solutions, stir at 25°C for 25 minutes to obtain a mixed solution; wherein, N-methyl-2-[4-dimethylamino]phenyl-3 , The volume ratio of 4-fullerenylpyrrolidine carbon tetrachloride solution to cetyltrimethylammonium bromide isopropanol solution is 1:3.5. After carrying out 4 times of solid-liquid separation and washing alternately to the mixed solution, it is placed at normal temperature and dried for 10 minutes; wherein, the solid-liquid separation process is centrifugation, and the rotating speed during centrifugation is 5000r / min, and the time is 10min, the washing treatment is to use ethanol to clean the solid matter obtained after centrifugation, and obtain such as figure 1 and figure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com