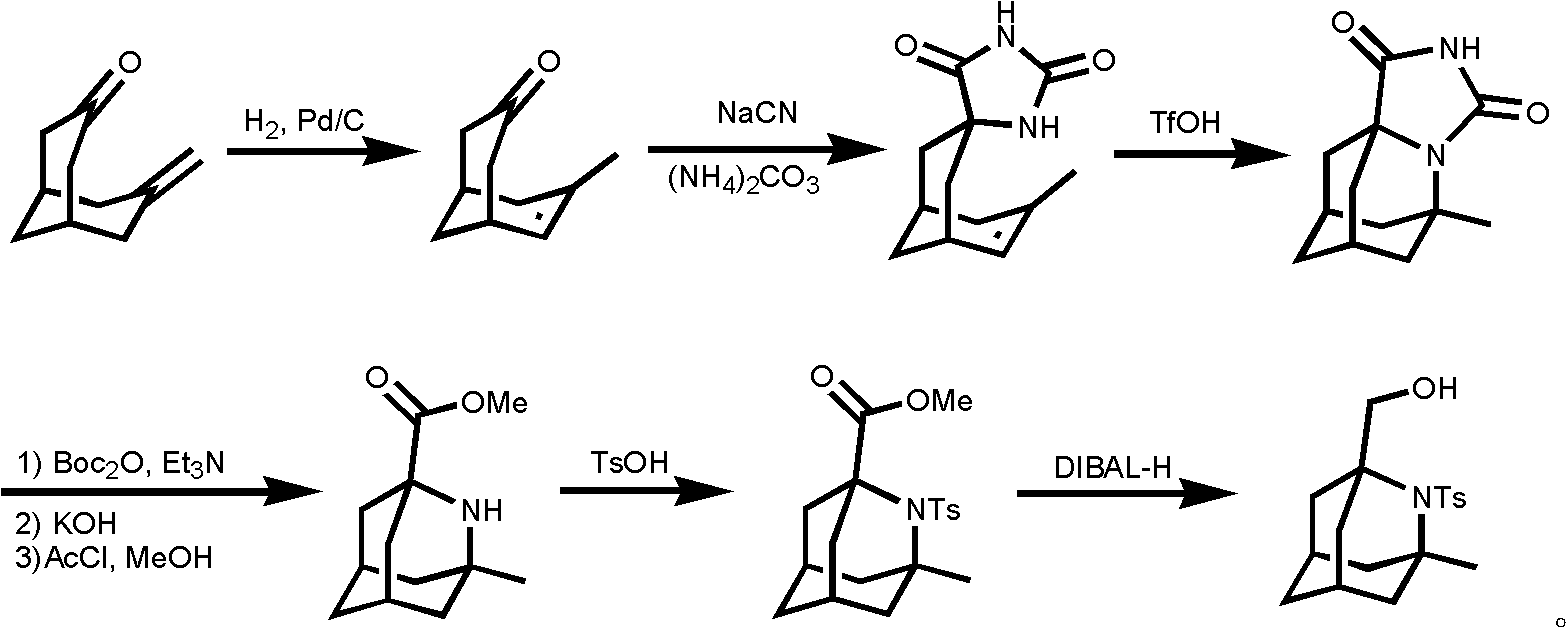
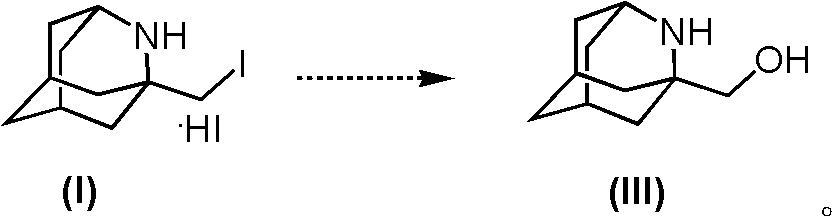
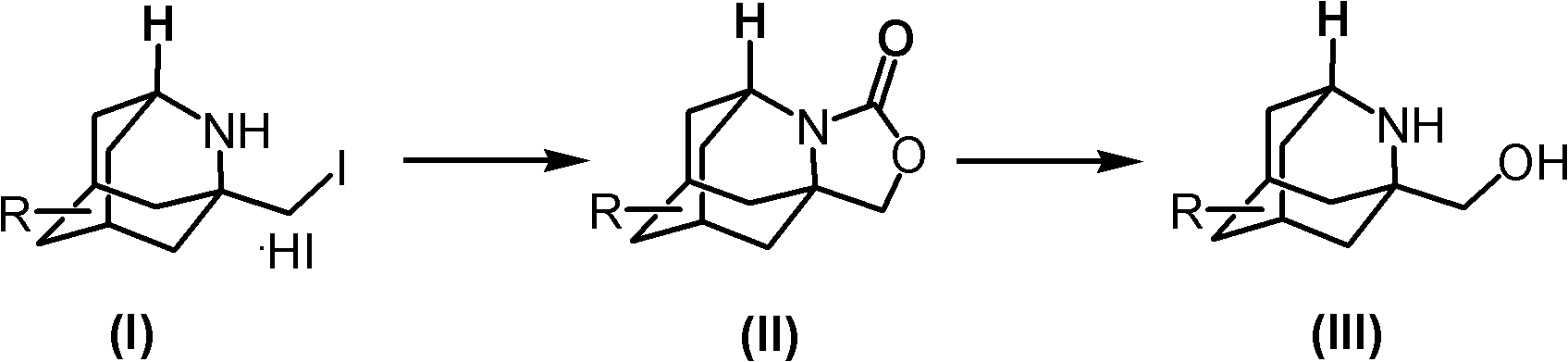
Method for synthesizing 1-hydroxymethyl-2-aza adamantane and derivatives thereof
An azaadamantane and hydroxymethyl technology, which is applied in the field of synthesizing 1-hydroxymethyl-2-azaadamantane and derivatives thereof, can solve the problems of lengthy routes, harsh deprotection conditions, and no compounds are obtained, Achieve the effects of cheap raw materials and reagents, mild reaction conditions, and novel preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1.1 Synthesis of 3,4-(2'-azaadamantane)oxazolin-2-one
[0024]
[0025] The compound 1-iodomethyl-2-azadamantane hydrogen iodide salt (8g, prepared according to the document J.Am.Chem.Soc., 2006, 128, 26, 8412-8413) was dissolved in 100mL of methanol, Di-tert-butyl dicarbonate (9 g) was added, and 1N aqueous sodium carbonate solution was added dropwise with stirring to control pH=9-10. Stir at room temperature for 3 hours, TLC detected that the reaction was complete, spin off the methanol under reduced pressure, extract with ethyl acetate (50mL*3), combine the organic phases and dry with sodium sulfate, filter, and concentrate to dryness to give compound 3,4- (2'-Azaadamantane)oxazolin-2-one (8 g, not purified) was directly used in the next reaction.
[0026] 1.2 Synthesis of 1-hydroxymethyl-2-azaadamantane
[0027]
[0028] The compound 3,4-(2'-azaadamantane)oxazolin-2-one was dissolved in 50% potassium hydroxide solution (150 mL), and heated to reflux overnigh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com