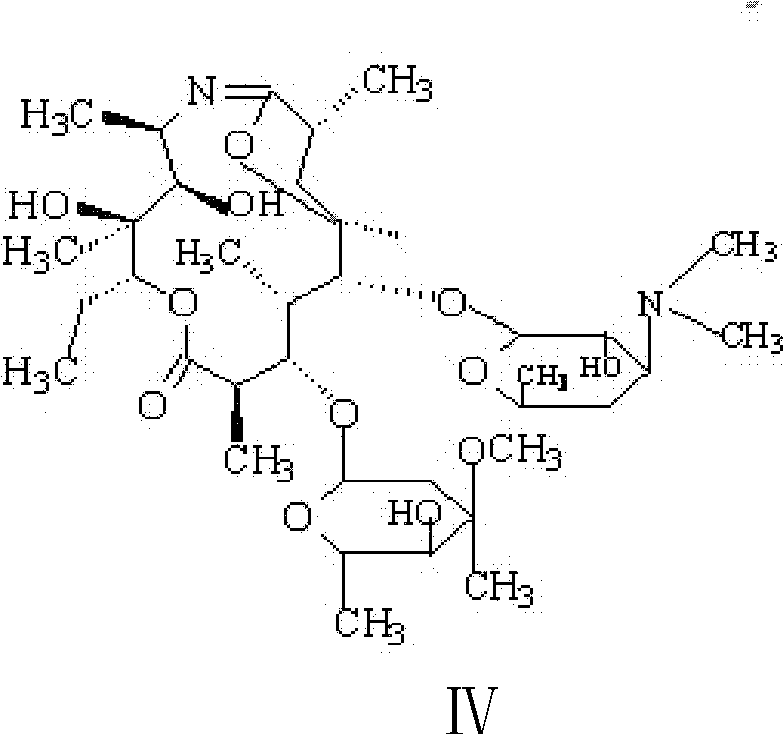
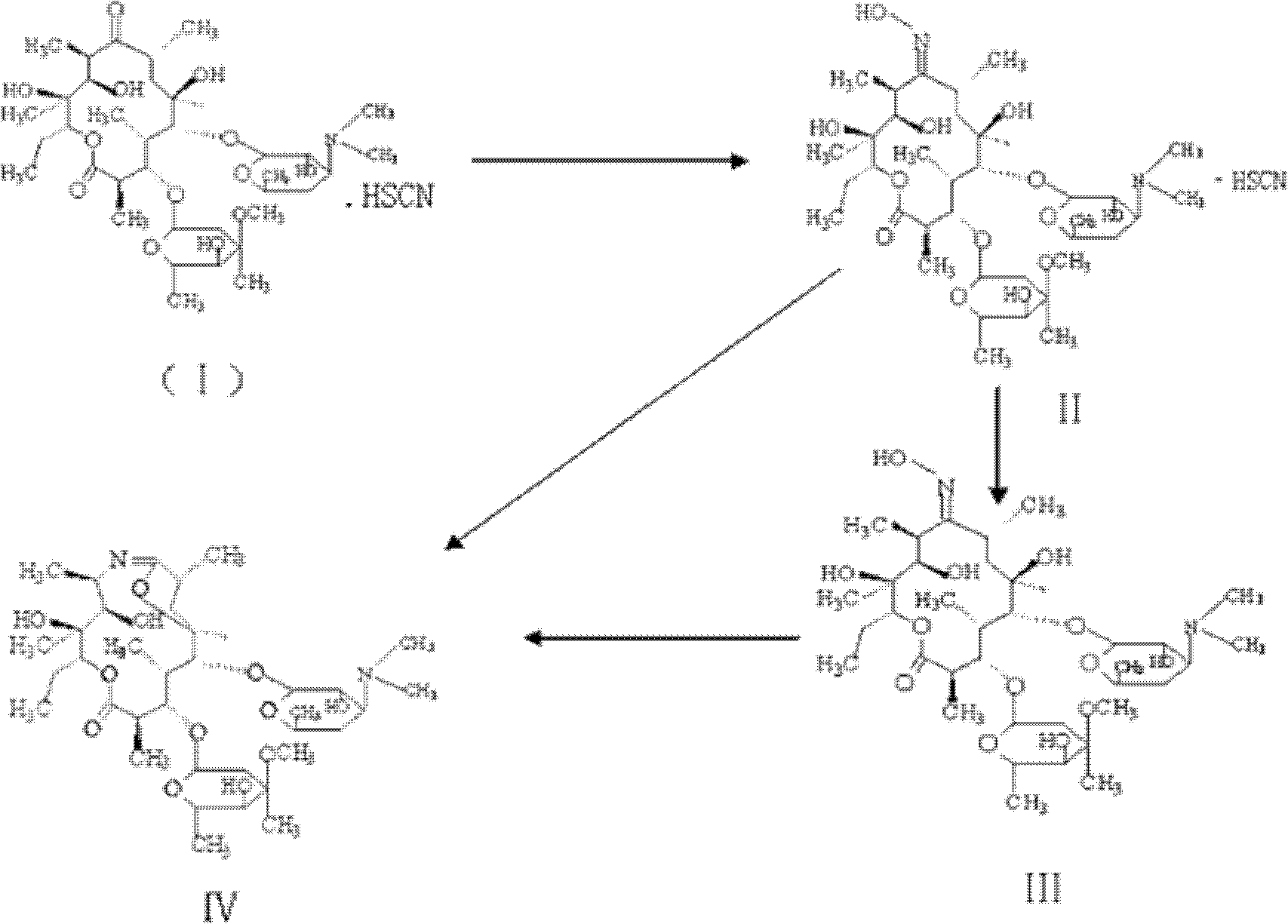
Preparation method of erythromycin 6,9 imino ether
A technology of erythromycin and imine ether, applied in the field of pharmaceutical preparation, can solve the problems of material loss, numerous operation steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Add 100g of methanol, 40g of hydroxylamine hydrochloride, 45g of sodium acetate, and 100g of erythromycin thiocyanate into a 500ml reaction bottle, heat up to 40-70°C, and react for 24-72 hours.
[0020] (2) HPLC monitors that the erythromycin content is less than 0.5%. After the reaction is over, add 100ml of deionized water dropwise, add 300ml of dichloromethane, add sodium hydroxide solution, adjust the pH=9 to 12, let stand to separate and discard water layer.
[0021] (3) Add 400ml of water and 20g of sodium bicarbonate to the dichloromethane layer, stir and cool down to 0-10°C, add the dichloromethane solution of p-toluenesulfonyl chloride (dissolve 40g of p-toluenesulfonyl chloride in 50ml of dichloromethane ), temperature control 0-10°C, reaction for 1.5-2.5 hours, acetic acid to adjust pH=5.0-6.0, standing to separate layers, adding sodium hydroxide solution to the water layer to adjust pH=9.0-12.0, filtering, drying at 60-90°C for 2 After ~5 hours, 74 g o...
Embodiment 2
[0023] (1) Add 110g of ethanol, 45g of hydroxylamine hydrochloride, 36g of sodium bicarbonate, and 100g of erythromycin thiocyanate into a 500ml reaction bottle, heat up to 40-70°C, and react for 24-72 hours.
[0024] (2) HPLC monitors that the erythromycin content is lower than 0.5%. After the reaction is over, add 100ml dropwise to drinking water, add 300ml dichloroethane, add sodium hydroxide solution, adjust pH=9~12, let stand for stratification, discard Remove the water layer.
[0025] (3) Add 300ml of water and 23g of sodium bicarbonate to the dichloroethane layer, stir and cool down to 0-10°C, add methanesulfonyl chloride in dichloroethane (32g of methanesulfonyl chloride dissolved in 40ml of dichloroethane middle), temperature control at 0-10°C, reaction for 1.5-2.5 hours, standing for stratification, adding sodium hydroxide solution to the water layer to adjust pH=9.0-12.0, filtering, drying at 60-90°C for 2-5 hours, and obtaining 6 , 9 imine ether 74g, HPLC purity 9...
Embodiment 3
[0027] (1) Add 110g of methanol, 42g of hydroxylamine hydrochloride, 36g of sodium bicarbonate, and 100g of erythromycin thiocyanate into a 500ml reaction flask, heat up to 40-70°C, and react for 24-72 hours.
[0028] (2) HPLC monitors that the content of erythromycin is less than 0.5%. After the reaction is over, add 300ml of chloroform and ammonia solution to adjust the pH to 9-11, let the mixture stand for separation, and discard the water layer.
[0029] (3) Add 400ml of water to the chloroform layer, heat up to 60-70°C, distill at normal pressure until the fraction temperature is 70-75°C, stop distillation, and cool down to room temperature.
[0030] (4) Add 300ml acetone and 15g sodium bicarbonate to the cooled reaction solution, stir and cool down to 0-10°C, add p-toluenesulfonyl chloride acetone solution (40g p-toluenesulfonyl chloride is dissolved in 120ml acetone), temperature control 0-10°C, react for 1.5-2.5 hours, add sodium hydroxide solution to adjust pH = 9.0-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com