Procambarus clarkia inhibin 1 gene as well as coded inhibin1 protein and application thereof
A technology of Procambarus clarkii and inhibitin, which is applied in the field of genetic engineering, and can solve problems such as the prohibitin gene and its application that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Cloning of the cDNA of Procambarus clarkii PHB1
[0057] 1) Extraction of total RNA: Total RNA was extracted using the existing technology one-step method (Trizol).
[0058] 2) cDNA first-strand synthesis: 5 microliters of total RNA, plus 1 microliter of SmartF (5'-TAC GGC TGC GAG AAG ACG ACA GAA GGG-3') and 1 microliter of Oligoanchor R (5'-GAC CAC GCG TAT CGA TGT CGA CT 16 (A / C / G)-3'), react at 72°C for 5 minutes, then add 5 times Buffer 4 microliters, dNTP2 microliters, RNase inhibitor 0.5 microliters, 1 microliter M-MLV reverse transcriptase, no 6.5 microliters of RNase-sterilized water were reacted at 42°C for 60 minutes, and the reaction was terminated at 70°C for 5 minutes.
[0059] 3) Amplification of the full-length cDNA of Procambarus clarkii PHB1
[0060] According to the conserved sequence of PHB1 in other species, the degenerate primer F1 was designed, and PHB1 was amplified by PCR with the universal back primer 3'primer:
[0061] Forward pri...
Embodiment 2
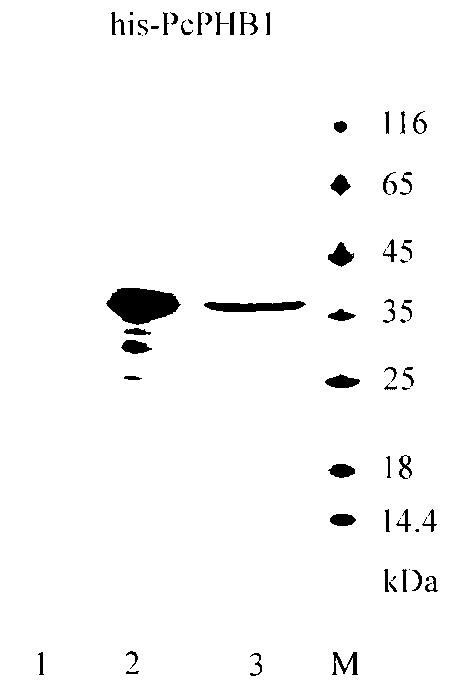
[0070] Example 2: Construction, expression and purification of prokaryotic recombinant expression vectors
[0071] (1) According to the sequence of Procambarus clarkii PHB1 and the cloning site of the expression vector pET30a (Novagen Company), design primers:
[0072] FcUbcExF:5ˊTAC TCA GAA TTC ATG GGG CAG ATT GGC TTC GGT3′(EcoR I)
[0073] FcUbc ExR:5ˊTAC TCA CTC GAG TCA CTG AGG AAG AGA TAA G3'(Xho I) (as shown in SEQ ID NO.5, 6)
[0074] When the primers are designed in the present invention, an EcoR I restriction site is introduced into the upstream primer, and an Xho I restriction site is introduced into the downstream primer.
[0075] (2) Gene amplification, cloning and recombinant plasmid screening
[0076] The liver cDNA was used as a template, and the above primers were used for PCR reaction. The amplification conditions were: 94°C, 3min pre-denaturation; 94°C, 30s, 53°C, 40s, 72°C, 50s, 35 cycles; 72°C, 10min extension.
[0077] 1% agarose gel electrophoresi...
Embodiment 3
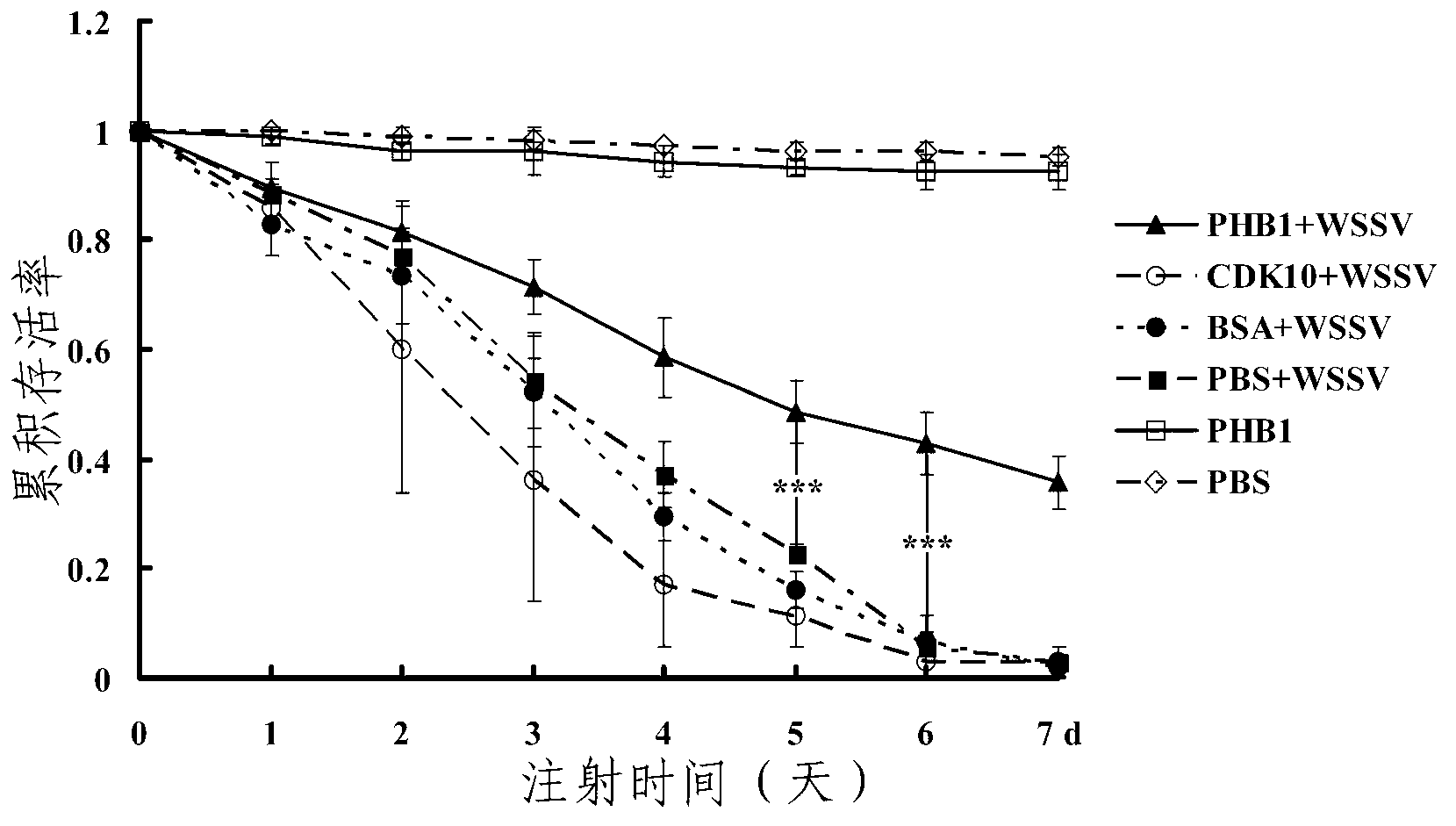
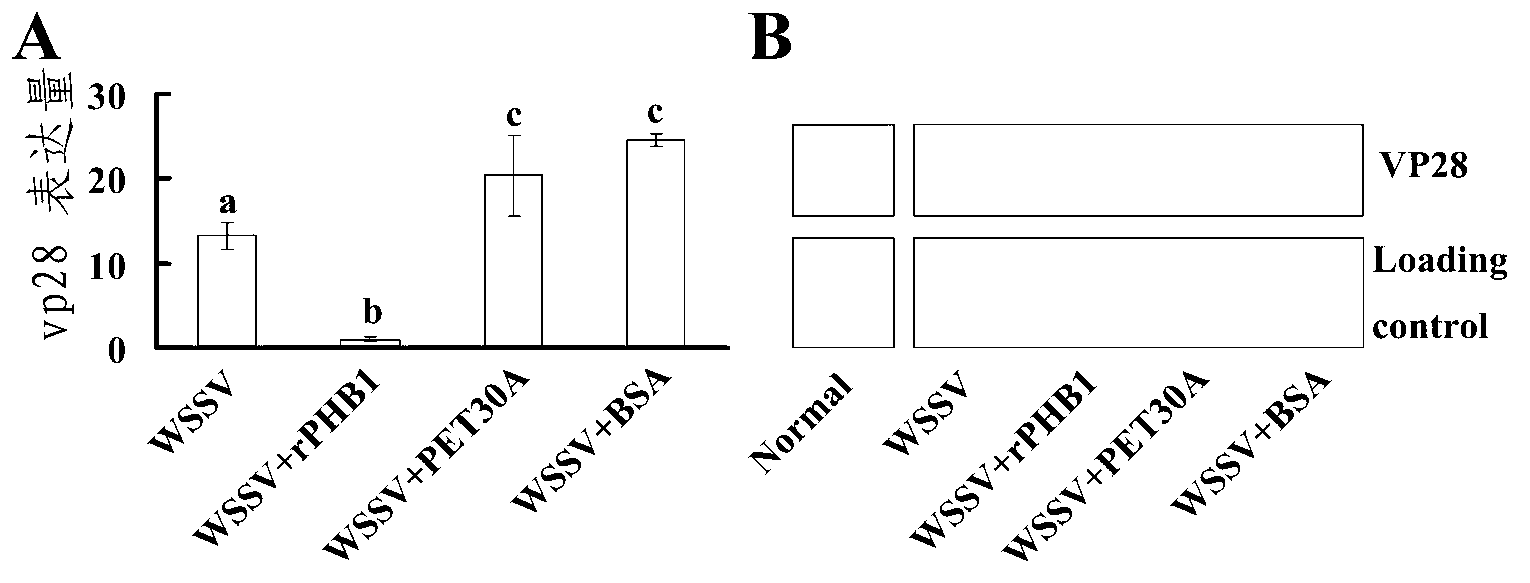
[0084] Example 3: Procambarus clarkii PHB1 recombinant protein has antiviral function
[0085] (1) Quantitative analysis of WSSV copy number by real-time quantitative PCR
[0086] Procambarus clarkii were randomly divided into 6 groups (30 in each group), in which the experimental group was injected with PBH1 co-incubated with white spot syndrome virus (WSSV), and 5 control groups were set up: respectively injected with ① CDK10 co-incubated with WSSV ( Protein recombinantly expressed in the same expression system as PHB1), ②bovine serum albumin (BSA)+WSSV, ③PBS+WSSV, ④inject PHB1 alone, ⑤inject PBS alone. Then the gill tissues of 3 Procambarus clarkii were randomly collected for genomic DNA extraction every day for 7 consecutive days. The gill tissue genome was extracted using the Genome Extraction Kit (MiniBEST Universal Genomic DNA Extraction Kit) from Bao Bioengineering Company (Dalian), and then diluted 10 times as a template for real-time quantitative PCR. Standard curv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com