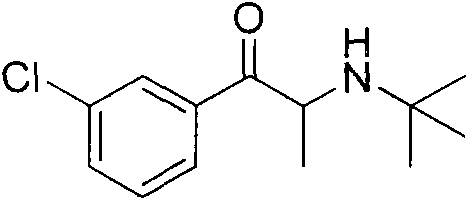
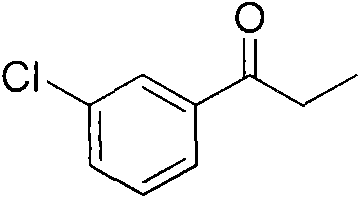
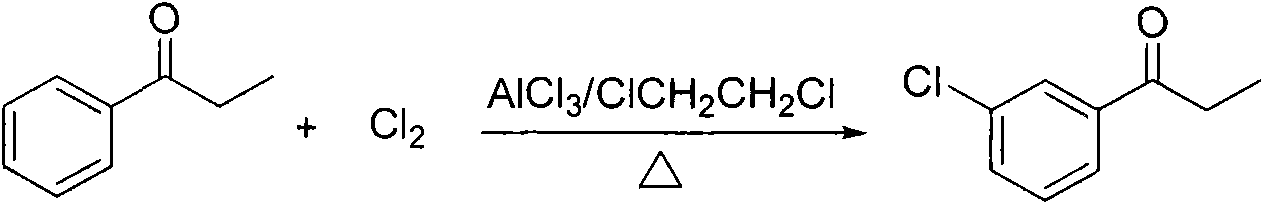Preparation method of amfebutamone intermediate m-chlorophenylacetone
A technology of chloropropiophenone and bupropion, applied in the field of medicine, can solve the problems of potential safety hazards, low reaction selectivity, etc., and achieve the effects of reducing damage, good catalytic activity, and controlling production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 100 kg of propiophenone into the reaction kettle, add 150 kg of anhydrous aluminum trichloride in 5 batches under slow stirring, and control the temperature at 20-25°C during the feeding process. After the completion of the feeding of aluminum trichloride, continue to insulate and stir for 0.5 hour to make the system uniform. Start to heat up, and when the temperature is 70°C, start to feed chlorine gas, and control the chlorine gas feed rate to 9kg / h. When the amount of feed reaches 55kg, HPLC will follow up and detect the reaction. When the remaining amount of propiophenone is less than 1.0%, stop feeding chlorine gas. The time of feeding chlorine gas is 6 hours, and the amount of chlorine gas is 60kg; cool to room temperature, and pour the reaction solution into 300kg In the crushed ice solution of hydrochloric acid, let stand to separate layers, remove the water phase, wash the organic phase with water, collect all fractions by distillation under reduced pressur...
Embodiment 2
[0035] Add 200kg of propiophenone into the reaction kettle, add 400kg of anhydrous aluminum trichloride in 6 batches under slow stirring, control the temperature not to exceed 25°C, continue to keep warm and stir for 1 hour after feeding the aluminum trichloride to make the system uniform. Slowly raise the temperature. When the temperature rises to 80°C, start to feed chlorine gas, and control the chlorine gas feed rate to 23kg / h. When the gas flow reaches 116kg, HPLC will track and detect the reaction. When the remaining amount of propiophenone is less than 1.0%, stop feeding Chlorine, the chlorine gas time is 5 hours. The amount of chlorine used is 136kg; cooled to room temperature, the reaction solution is poured into 800kg hydrochloric acid crushed ice solution, allowed to stand for stratification, the water phase is removed, the organic phase is washed with water, the pressure is 400Pa, and the temperature is reduced pressure distillation at 135°C to collect all Distillat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com