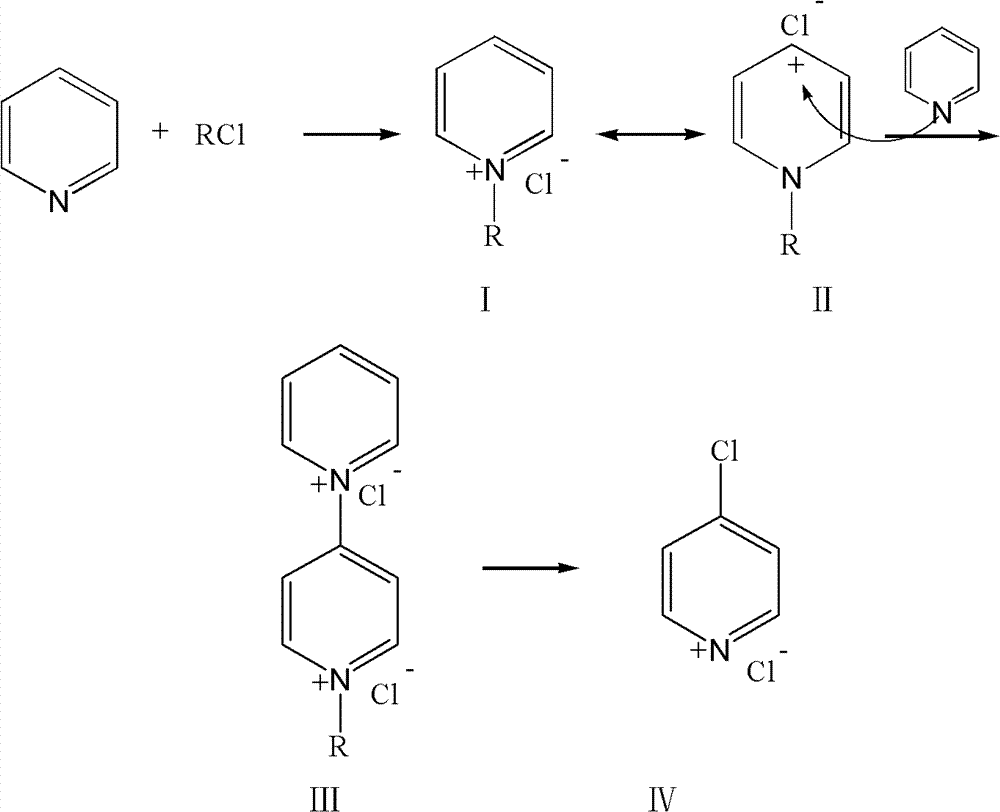
Method for synthesizing 4-chloro-pyridine
A synthetic method, the technology of chloropyridine, which is applied in the field of synthesis of pharmaceutical and pesticide intermediates, can solve the problems of few manufacturers in the research of 4-chloropyridine synthesis, affect the continuous production of industrial reactions, and blockage of reactors, etc., and achieve the solution of pyridine coking , easy to industrialized production, the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Starting material: pyridine; organic solvent: ethyl acetate; chlorination reagent: thionyl chloride
[0032] Add 200g of anhydrous pyridine and 100-200mL of ethyl acetate in sequence in a 1000mL reaction flask, protect with nitrogen, cool in a cold water bath, slowly add 200g of thionyl chloride dropwise under stirring, and the temperature does not exceed 40°C. After the addition, the temperature was raised and kept at 70-75° C. for 5 hours. During this period, the color of the reaction mixture gradually became darker, and finally turned into a black oily substance. Cool down and let it stand, add 500ml ethanol to the reaction solution, heat it at 50-60°C and stir for 1 hour, cool down to about 10°C, filter, it is easier to filter with suction, the filtrate is dark, the solid is dark brown, soak in absolute ethanol Wash and dry to obtain 266.8 grams of tan solid powder, with a yield of about 70.2%. Its purity was 95.6% as determined by HPLC. The product was identifie...
Embodiment 2
[0034] Starting material: pyridine; Organic solvent: dichloromethane; Chlorinating reagent: phosphorus oxychloride
[0035] Add 200g of anhydrous pyridine and 150mL of dichloromethane to a 1000mL reaction flask in sequence, cool in a cold water bath, slowly add 160g of phosphorus oxychloride dropwise under stirring, and the temperature does not exceed 50°C. After the addition, the temperature was raised and kept at 70-75° C. for 5 hours. During this time, the color of the reaction mixture gradually became darker. Cool down and stand still, add 500ml ethanol to the residue in the lower layer, heat at 50-60°C and stir for 1 hour, cool down to about 10°C, filter, easy to filter with suction, the filtrate is dark, the solid is dark brown, use absolute ethanol Soak, wash, and dry to obtain 152 grams of tan solid powder, with a yield of about 52.4%. Its purity was 93.9% as determined by HPLC. The product was identified as 4-chloropyridine hydrochloride by NMR identification. The...
Embodiment 3
[0037] Starting material: pyridine; organic solvent: chlorobenzene; chlorination reagent: phosphorus pentachloride
[0038] Add 100g of anhydrous pyridine and 100mL of chlorobenzene in sequence in a 500mL reaction bottle, protect with nitrogen, cool in a cold water bath, slowly add 100g of phosphorus pentachloride dropwise under stirring, and the temperature does not exceed 30°C. After the addition, the temperature was raised and kept at 70-75° C. for 5 hours. During this time, the color of the reaction mixture gradually became darker. Cool down and let it stand, add 300ml n-butanol to the residue in the lower layer, heat it at 50-60°C and stir for 1 hour, cool down to about 10°C, filter, it is easier to filter with suction, the filtrate is dark, the solid is dark brown, dry it 116.2 g of tan solid powder was obtained, with a yield of about 61.3%. Its purity was 92.7% as determined by HPLC. The product was identified as 4-chloropyridine hydrochloride by NMR identification. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com