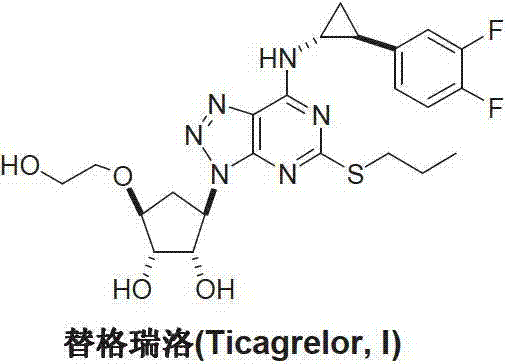
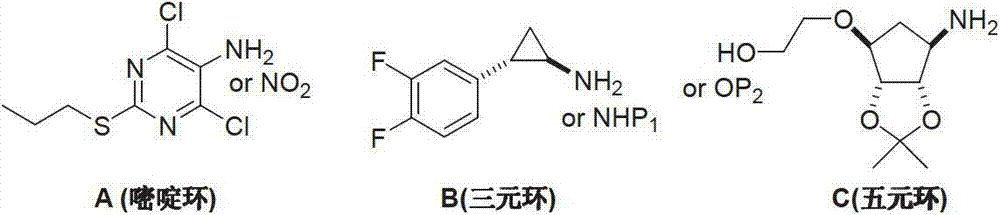
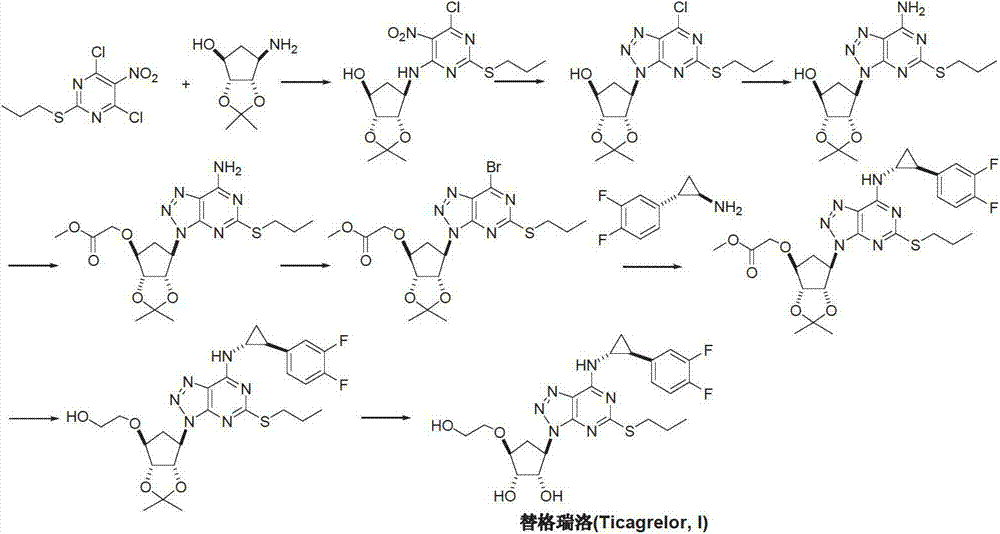
Method for preparing ticagrelor
A technology of ticagrelor and dimethyl, which is applied in the field of preparation of the new anticoagulant drug ticagrelor, can solve the problem of difficult control of the coupling position, avoid the use of dangerous chemicals, and make the preparation process fast and convenient. Suitable for large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 1-[(3aR, 4S, 6R, 6aS)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxol-4 to the reaction flask -Oxy]ethyl acetate]-6-yl]-5-amino-4-carboxamido-1,2,3-triazole (II) (3.69g, 10mmol), thiourea (1.0g, 13mmol ) and toluene 25mL. Slowly raise the temperature to 100° C., and react for 10 hours under the protection of nitrogen. The solvent was removed under reduced pressure to give oil 9-[(3aR,4S,6R,6aS)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadieno-1,3-dioxane Pent-4-oxyl]ethyl acetate]-6-yl]-2-thio-6-oxo-8-azapurine (or named: 2-{[(3aR, 4S, 6R, 6aS) -6-{7-oxo-5-oxo-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl}-2,2-dimethyl-tetrahydro -3aH-cyclopentadiene[d][1,3]-dioxol-4-yl]oxy}ethyl acetate) (III) 3.5g, yield 85.2% (can be directly used in the following step reaction).
Embodiment 2
[0038] Add 9-[(3aR, 4S, 6R, 6aS)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxol-4 to the reaction flask -Oxy]ethyl acetate]-6-yl]-2-thioxo-6-oxo-8-azapurine (III) (2.10g, 5mmol), potassium hydroxide solution (0.1M, 10mL) and Acetonitrile 25mL, room temperature was added dropwise bromopropane (1.53g, 12.5mmol) acetonitrile solution. The reaction was stirred at room temperature for 15 hours. The solvent was recovered under reduced pressure, the residue was extracted 3 times with dichloromethane, the organic phases were combined, dried, and distilled under reduced pressure to obtain the oil 9-[(3aR, 4S, 6R, 6aS)-[[2,2-dimethyl- Tetrahydro-4H-cyclopentadieno-1,3-dioxol-4-oxy]ethyl acetate]-6-yl]-2-propylmercapto-6-oxo-8-aza Purine (or named: 2-{[(3aR, 4S, 6R, 6aS)-6-{7-oxo-5-propylmercapto-3H-[1,2,3]triazolo[4,5- d]pyrimidin-3-yl}-2,2-dimethyl-tetrahydro-3aH-cyclopentadieno[d][1,3]-dioxol-4-yl]oxy}acetic acid Ethyl ester) (IV) 2.1g, yield 92.7%.
Embodiment 3
[0040] Under nitrogen protection, add 9-[(3aR, 4S, 6R, 6aS)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxa Cyclopent-4-oxyl]ethyl acetate]-6-yl]-2-propylmercapto-6-oxo-8-azapurine (IV) (1.13g, 2.5mmol), benzotriazole- 1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (BOP) (1.7 g, 3.75 mmol) and acetonitrile 25 mL. Under stirring, 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) (0.6 g, 3.75 mmol) was added and reacted at room temperature for 12 hours. The temperature was raised to 60° C., and the reaction was continued for 12 hours. The solvent was distilled off under reduced pressure, dissolved in 25 mL of ethyl acetate, and washed with 10 mL of 2M sodium hydroxide. The organic phase was separated, dried and concentrated under reduced pressure. The residue was dissolved in 25 mL of tetrahydrofuran, and trans-(1R, 2S)-2-(3,4-difluorophenyl) cyclopropylamine (0.5 g, 3 mmol) and sodium hydride (0.1 g, 4 mmol) were added, and the temperature was raised to 50° C., st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com