Aryne derivatives as protein kinase inhibitors and their medical use
A drug and compound technology, applied in the field of protein kinase inhibitor aryne derivatives, can solve the problem of imatinib not being able to combine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
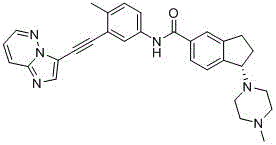
[0128] Example 1. (R)-N-(3-(imidazo[1,2-b]pyridazin-3-yl)ethynyl)4-methylphenyl)-1-(4-methylpiperazine -1-yl)-2,3-dihydro-1H-indene-5-carboxamide (Compound 1)
[0129]
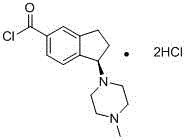
[0130] Step 1 Synthesis of (R)-1-(4-methylpiperazin-1-yl)-2,3-dihydro-1H-indene-5-carbonyl chloride hydrochloride
[0131]
[0132] (R)-1-(4-methylpiperazin-1-yl)-2,3-dihydro-1H-indene-5-carboxylic acid hydrochloride (prepared according to the synthesis method disclosed in CN101759683A) 3.0g Dissolve in 30ml of dichloromethane, then add 4.2ml of thionyl chloride and a few drops of DMF dropwise, after heating to reflux for 2 hours, a large amount of solids precipitated, filtered, and the filter cake was washed several times with dichloromethane to obtain 4.5g of white solid .
[0133] Step 2 (R)-N-(3-iodo-4-methylphenyl)-1-(4-methylpiperazin-1-yl)-2,3-dihydro-1H-indene-5-methanol Amide synthesis
[0134]
[0135] 6.7 g of 3-iodo-4-methylaniline hydrochloride was dissolved in dry dichloromethane, an...
Embodiment 2
[0140] Example 2. (S)-N-(3-(imidazo[1,2-b]pyridazin-3-yl)ethynyl)4-methylphenyl)-1-(4-methylpiperazine -1-yl)-2,3-dihydro-1H-indene-5-carboxamide (Compound 2)
[0141]
[0142] Step 1 Synthesis of (S)-1-(4-methylpiperazin-1-yl)-2,3-dihydro-1H-indene-5-carbonyl chloride hydrochloride
[0143]
[0144] (S)-1-(4-methylpiperazin-1-yl)-2,3-dihydro-1H-indene-5-carboxylic acid hydrochloride (prepared according to the synthesis method disclosed in CN101759683A) 4.0g Dissolve in 25ml of dichloromethane, then add 4.6ml of thionyl chloride and a few drops of DMF dropwise, after heating to reflux for 2 hours, a large amount of solids precipitated, filtered, and the filter cake was washed several times with dichloromethane to obtain 3.5g of white solid .
[0145] Step 2 (S)-N-(3-iodo-4-methylphenyl)-1-(4-methylpiperazin-1-yl)-2,3-dihydro-1H-indene-5-methanol Amide synthesis
[0146]
[0147] Dissolve 13.4 g of 3-iodo-4-methylaniline hydrochloride in dry dichloromethane, then a...
Embodiment 3
[0151] Example 3. (R)-N-(3-((1H-pyrazol[3,4-b]pyridin-5-yl)ethynyl)-4-methylphenyl)-1-(4-methyl (Piperazin-1-yl)-2,3-dihydro-1H-indene-5-carboxamide (Compound 3)
[0152]
[0153] Step 1: Synthesis of 5-((trimethylsilyl)ethynyl)-1H-pyrazolo[3,4-b]pyridine
[0154]
[0155] Dissolve 3.0g of 5-bromo-1H-pyrazol[3,4-b]pyridine in DMF, then add 2.98g of trimethylsilylacetylene, 0.53g of Pd(dppf) 2 Cl 2 , 0.29g cuprous iodide and 3.86g triethylamine were added to the solution, the air was removed, and N 2 Protection, heated to 70 ° C for about 2h to complete the reaction. Extract, dry over anhydrous sodium sulfate, filter and spin dry to obtain a crude product. Silica gel column chromatography was used for gradient elution with ethyl acetate:petroleum ether=1:20-2:1 to obtain 3.5 g of the title compound.
[0156] Step 2: Synthesis of 5-ethynyl-1H-pyrazol[3,4-b]pyridine
[0157]
[0158] Dissolve 4.5g of 5-((trimethylsilyl)ethynyl)-1H-pyrazol[3,4-b]pyridine in methanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com