Puerarin derivatives and their synthetic methods and applications
A technology of puerarin derivatives and synthesis methods, which can be used in drug combinations, medical preparations containing active ingredients, and pharmaceutical formulations, etc., can solve the problem of low selectivity of pharmacological targets, improve learning and memory disorders, balance internal and external The effect of osmotic pressure and improving fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0039] Specific embodiment one: in this embodiment, puerarin derivatives are represented by the following general formula (I):
[0040]
[0041] (I), where R 1 is hydrogen, cyano, cyanide, ethyl acetate or acetamide, R 2 is hydrogen, cyano, cyanide, ethyl acetate or acetamide, R 1 , R 2 Not simultaneously hydrogen.
[0042] The puerarin derivatives described in this embodiment are mainly produced by chemical synthesis methods such as addition reaction.
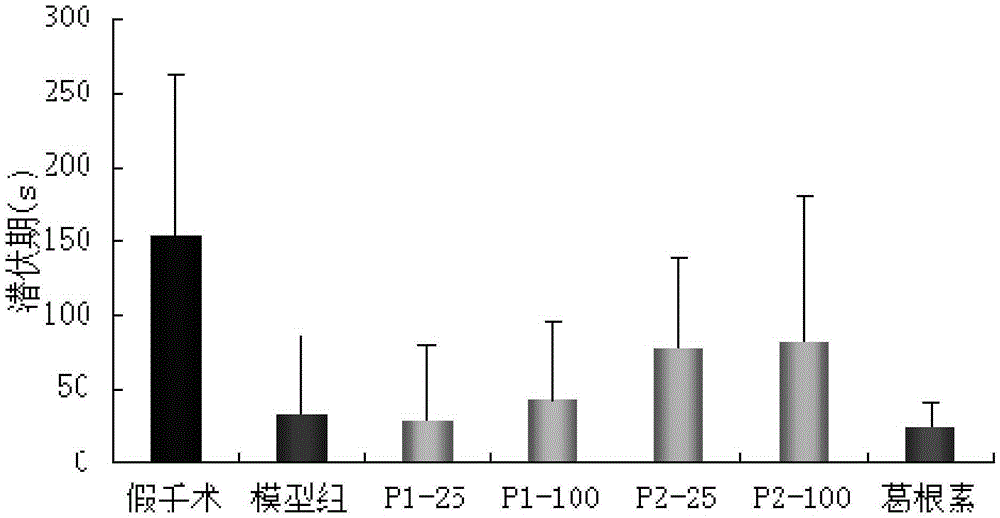
[0043] The puerarin derivatives described in this embodiment are used in the treatment of vascular dementia.
specific Embodiment approach 2
[0044] Specific embodiment two: the present embodiment puerarin derivative is represented by following structural formula (II):
[0045]
[0046] (II), its synthetic method is carried out according to the following steps:
[0047] Dissolve 36mmol puerarin in 200mL of absolute ethanol, add 216.3mmol K 2 CO 3 , stirred for 80min, added 216mmol chloroacetonitrile, stirred at room temperature for 10h, filtered (removed solid insolubles), rotary steamed into oil, ground with anhydrous ether, poured off anhydrous ether, spin-dried, dissolved with anhydrous methanol, and then used The eluate with a volume ratio of dichloromethane to methanol of 40:1-15:1 was subjected to silica gel column chromatography to obtain a puerarin derivative of structural formula (II).
[0048] The puerarin derivatives described in this embodiment are used in the treatment of vascular dementia.
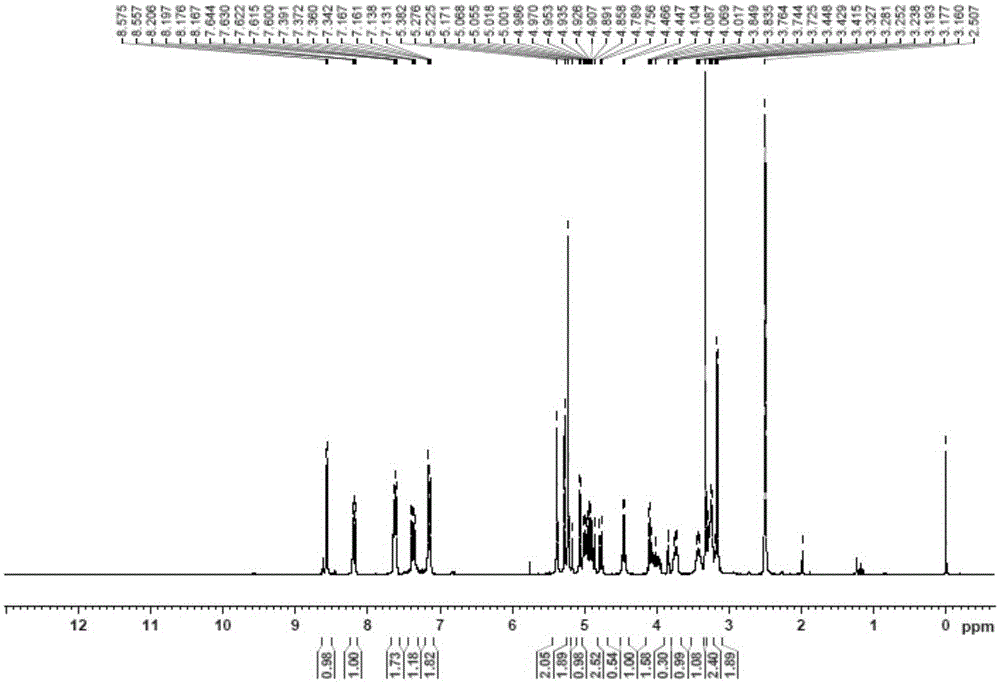
[0049] The proton nuclear magnetic resonance spectra of the puerarin derivatives of structural formula (Ⅱ)...
specific Embodiment approach 3
[0050] Specific embodiment three: the puerarin derivative of this embodiment is represented by the following structural formula (Ⅲ):
[0051]
[0052] (Ⅲ), its synthetic method is to carry out according to the following steps:
[0053] Dissolve 36mmol of puerarin in 200mL of anhydrous DMF, add 216.3mmol of NaOH at room temperature, stir for 60min, add 216mmol of chloroacetonitrile, stir for 10h at room temperature, filter (remove solid insolubles), rotary steam to oil, grind with anhydrous acetone, pour The solvent was removed from anhydrous acetone, spin-dried, dissolved in anhydrous methanol, and separated by silica gel column chromatography with an eluent with a volume ratio of dichloromethane to methanol of 15:1 to 10:1 to obtain a puerarin derivative of structural formula (Ⅲ).
[0054] The puerarin derivatives described in this embodiment are used in the treatment of vascular dementia.
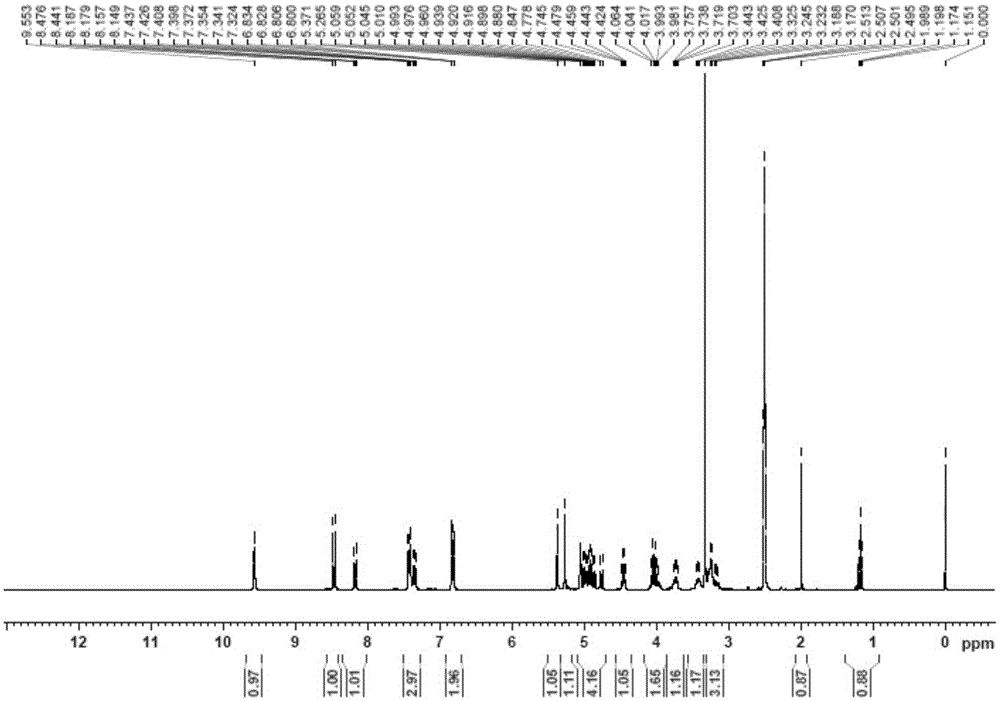
[0055] The proton nuclear magnetic resonance spectra of structural formula (Ⅲ) pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com