Lumbrokinase enteric-coated tablet and preparation method thereof
A lumbrokinase entero and lumbrokinase technology, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of low probability of capsules, increased pollution, and high product cost, and achieves The quality and curative effect are stable and reliable, the effect of overcoming the reduction of potency and overcoming technical defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
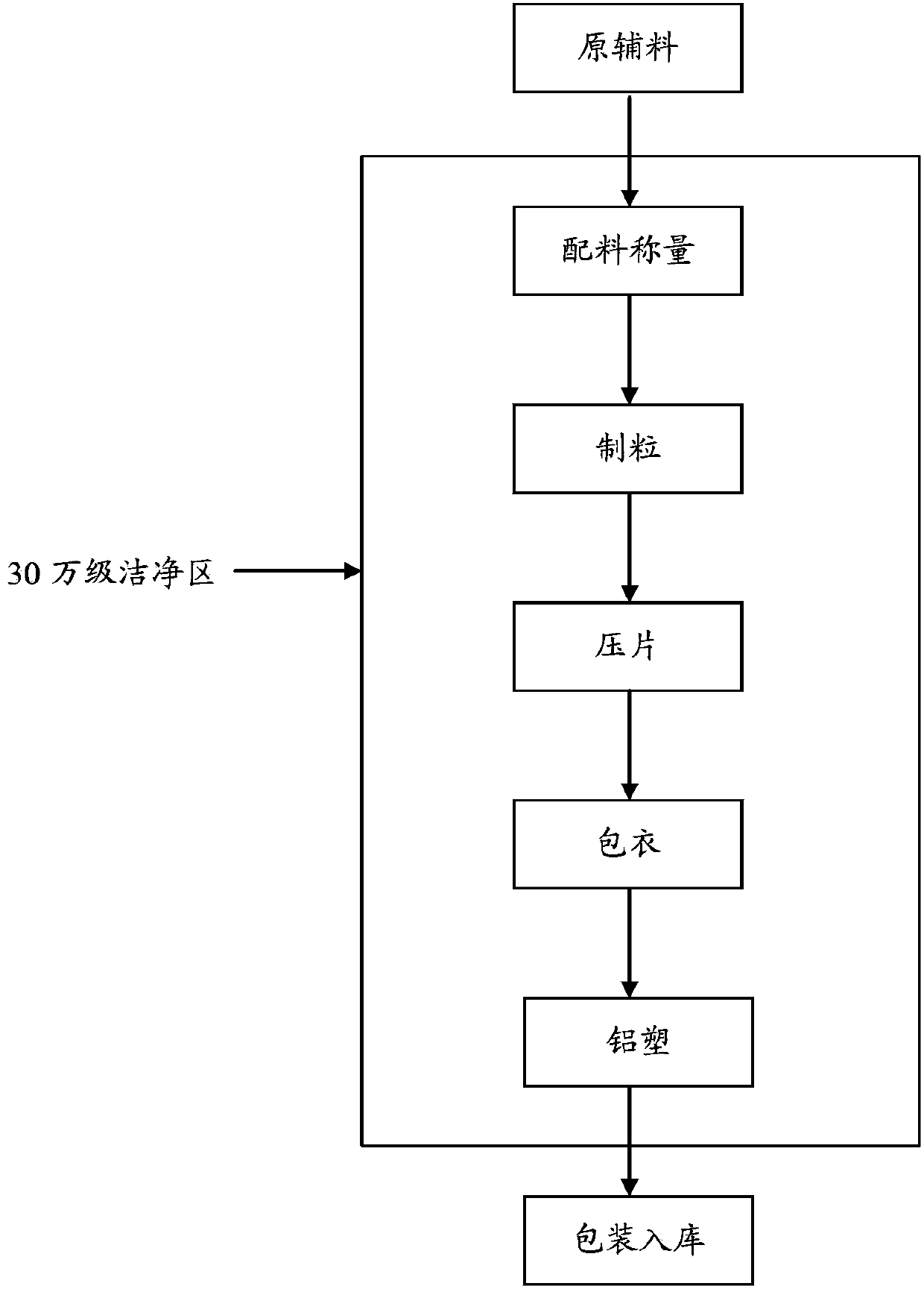
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 lumbrokinase enteric-coated tablets
[0031] 1. Prescription (600,000 tablets):
[0032]
[0033] 2. Preparation process
[0034] 1. wet granules
[0035] All raw and auxiliary materials must reach 80-mesh fineness, sucrose is crushed with a universal grinder, and passed through a 80-mesh sieve. Prepare wet granules in four batches, each batch of raw and auxiliary materials is: 45 billion units of lumbrokinase raw material powder (about 2.5kg), 3.75kg of hydroxypropyl cellulose, 30.45kg of sucrose, 0.575kg of sodium carboxymethyl starch, Put 1.9kg of talcum powder in the mixer of the high-speed mixing granulator, turn on the stirring paddle (1500 rpm) and mix for 15 minutes; add 15kg of 50% (volume ratio) ethanol aqueous solution, turn on the stirring paddle (1500 rpm) and mix for 2 ~7 minutes, then turn on the stirring paddle (3000 rpm) and the granulating paddle (3000 rpm) to mix and granulate for 1-2 minutes; open the discharge v...
Embodiment 2
[0113] Preparation of embodiment 2 lumbrokinase enteric-coated tablets
[0114] 1. Prescription (600,000 tablets):
[0115]
[0116] 2. Preparation process
[0117] 1. wet granules
[0118] All raw and auxiliary materials must reach 80-mesh fineness, sucrose is crushed with a universal grinder, and passed through a 80-mesh sieve. Prepare wet granules in four batches, each batch of raw and auxiliary materials: Lumbrokinase raw material powder 45 billion units (about 2.5kg), hydroxypropyl cellulose 3.75kg, sucrose 30.45kg, sodium carboxymethyl starch 0.575kg, talc Put 1.9kg of powder into the mixer of the high-speed mixing granulator, turn on the stirring paddle (1500 rpm) and mix for 15 minutes; add 15kg of 50% (volume ratio) ethanol aqueous solution, turn on the stirring paddle (1500 rpm) and mix for 2~ After 7 minutes, turn on the stirring paddle (3000 rpm) and the granulating paddle (3000 rpm) to mix and granulate for 1 to 2 minutes; open the discharge valve and discha...
Embodiment 3
[0136] The preparation of embodiment 3 lumbrokinase enteric-coated tablets
[0137] 1. Prescription (600,000 tablets):
[0138]
[0139]
[0140] 2. Preparation process
[0141] 1. wet granules
[0142] All raw and auxiliary materials must reach 80-mesh fineness, sucrose is crushed with a universal grinder, and passed through a 80-mesh sieve. Prepare wet granules in four batches, each batch of raw and auxiliary materials: Lumbrokinase raw material powder 45 billion units (about 2.50kg), hydroxypropyl cellulose 3kg, sucrose 36.54kg, carboxymethyl starch sodium 0.575kg, talc powder 2.28kg, put it in the mixer of the high-speed mixing granulator, turn on the stirring paddle (1500 rpm) and mix for 15 minutes; add 15kg of ethanol (50%) aqueous solution, turn on the stirring paddle (1500 rpm) and mix for 2 to 7 minutes , and then turn on the stirring paddle (3000 rpm) and the granulating paddle (3000 rpm) to mix and granulate for 1 to 2 minutes; open the discharge valve and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com