A method for preparing tissue-engineered spinal cord using dermal-derived mesenchymal stem cells
A technology for tissue engineering spinal cord and cells, applied to cells modified by introducing foreign genetic material, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problem of poor repair effect, difficult to achieve neural stem cells and embryonic stem cells Issues such as autologous transplantation and improper transplantation timing can achieve the effect of promoting cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Isolation of dMSCs
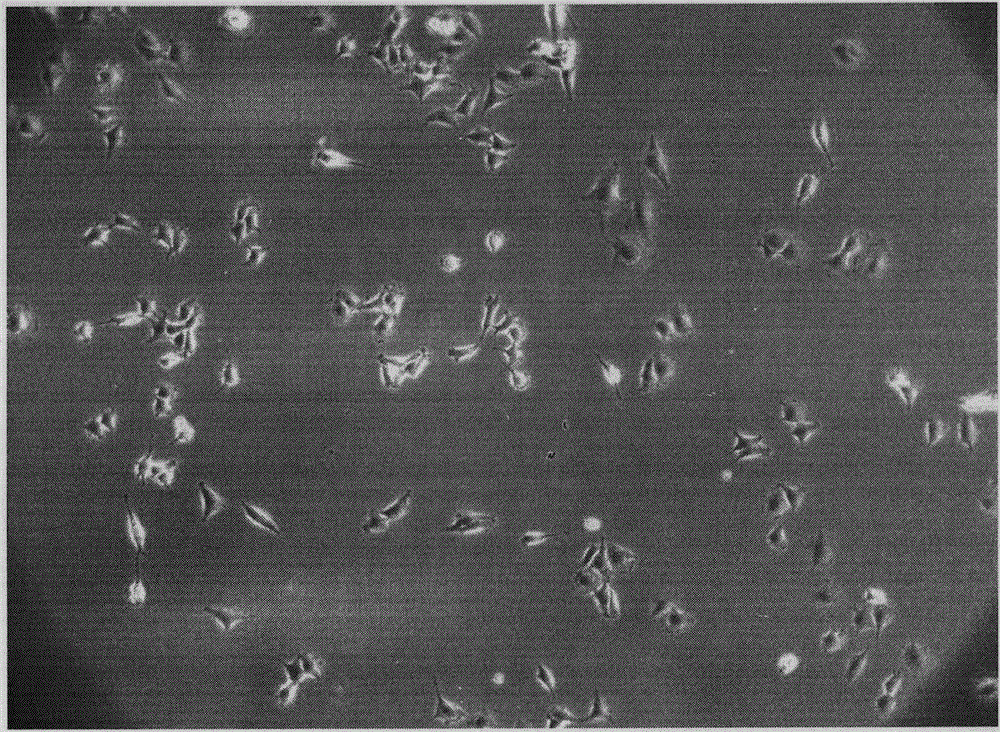
[0026] After routine disinfection of the inner thigh or back skin of patients with spinal cord injury, anesthetized with 1% lidocaine, the size is about 0.5×4cm 2 full-thickness skin. Alcohol disinfection, cut the skin and put it in 0.25% trypsin to digest overnight. The next day, after washing with Hank's solution, the epidermis and subcutaneous tissue were removed, and the dermal tissue was cut into cell fragments and blown into a cell suspension, which was filtered and inoculated into a culture bottle, and the medium was IMEM / 10% fetal bovine serum. After 6 hours, discard the culture medium and suspended cells, and continue to culture the early adherent cells. Passage repeatedly until colony-like cell populations are formed, inoculate and select cell clones according to conventional methods, and passage for future use. Isolated dMSCs such as figure 1 shown.
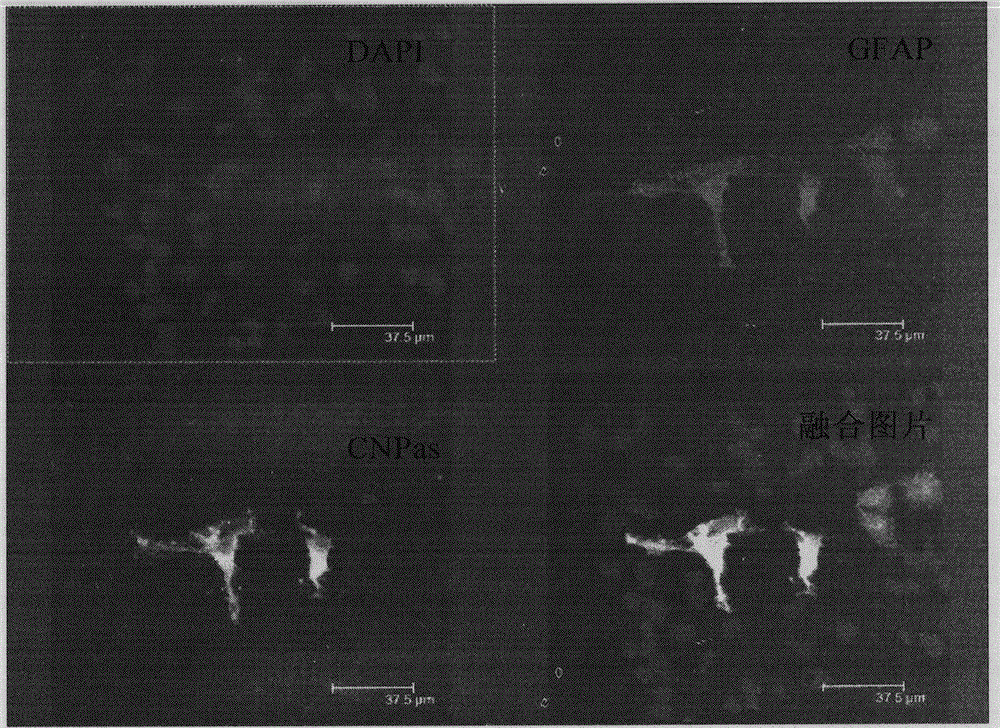
[0027] Among them, cell immunofluorescence histochemistry was used to det...
Embodiment 2d
[0028] Embodiment 2d MSCs rapid expansion
[0029] Usually, the growth of primary cells is relatively slow. In order to obtain a sufficient amount of cells within the optimal time window for transplantation, this example studies the promotion of proliferation of dMSCs by different growth factors. According to literature research, a variety of cytokines are involved in the regulation of stem cell proliferation, mainly basic fibroblast growth factor (basic fibroblast growth factor-2, FGF-2), epidermal growth factor (epidermal growth factor, EGF), leukemia inhibitory factor (1eukemia inhibiting factor, LIF), db-cAMP, insulin growth factor-1 (insulin growth factor-1, IGF-1), insulin growth factor-2 (insulin growth factor-2, IGF-2), platelet-derived growth factor (Plate derived growth factor, PDGF), brain derived neurotrophic factor (brain derived neurotrophic factor, BDNF), neurotrophin-3 (neurotrophin-3, NT-3), nerve growth factor-B (nerve growth factor-β, NGF -β), bone morphoge...
Embodiment 3d
[0032] Example 3d Optimization of MSCs differentiation neuron and oligodendrocyte differentiation conditions
[0033] The local microenvironment of spinal cord injury is not conducive to the differentiation of transplanted stem cells into neurons and other repair cells. Therefore, it is necessary to first explore the conditions that increase the differentiation of stem cells into neurons and other repair cells, and then combine these conditions in the tissue engineered spinal cord to improve stem cell transplantation. repair effect.
[0034] In vitro experiments were performed to observe the conditions for the differentiation of dMSCs into neurons and oligodendrocytes. There are three kinds of media for inducing neuron differentiation: (1) 5% FBS; (2) 6ng / ml retinoic acid; (3) 10ng / ml BDNF+10ng / mlNT3+6ng / ml retinoic acid+5%FBS. Two kinds of induction media were used for induction to Schwann cells: (1) 5% FBS; (2) 5% FBS+10 ng / ml Neuregulin. The results showed that the probab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com