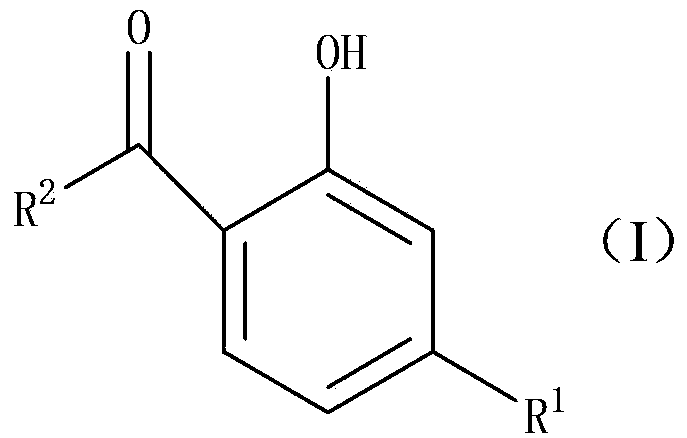
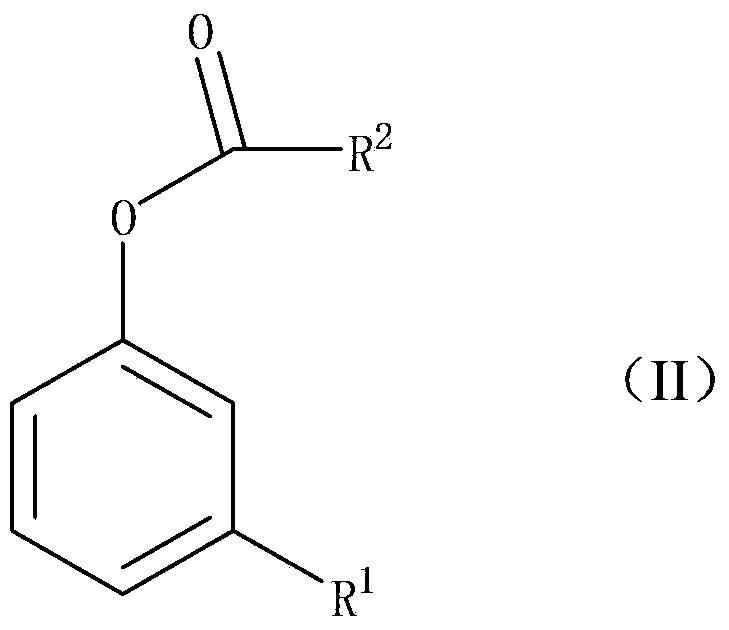
Preparation method of 2-hydroxyl-4-substituted arone compound
A technology for aryl ketones and compounds, which is applied in the field of preparation of 2-hydroxy-4-substituted aryl ketone compounds, can solve the problems of unsuitability for large-scale industrial production, difficulty in product separation and purification, and excessive production of three wastes, etc., and achieve excellent solubility properties , low process pollution and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
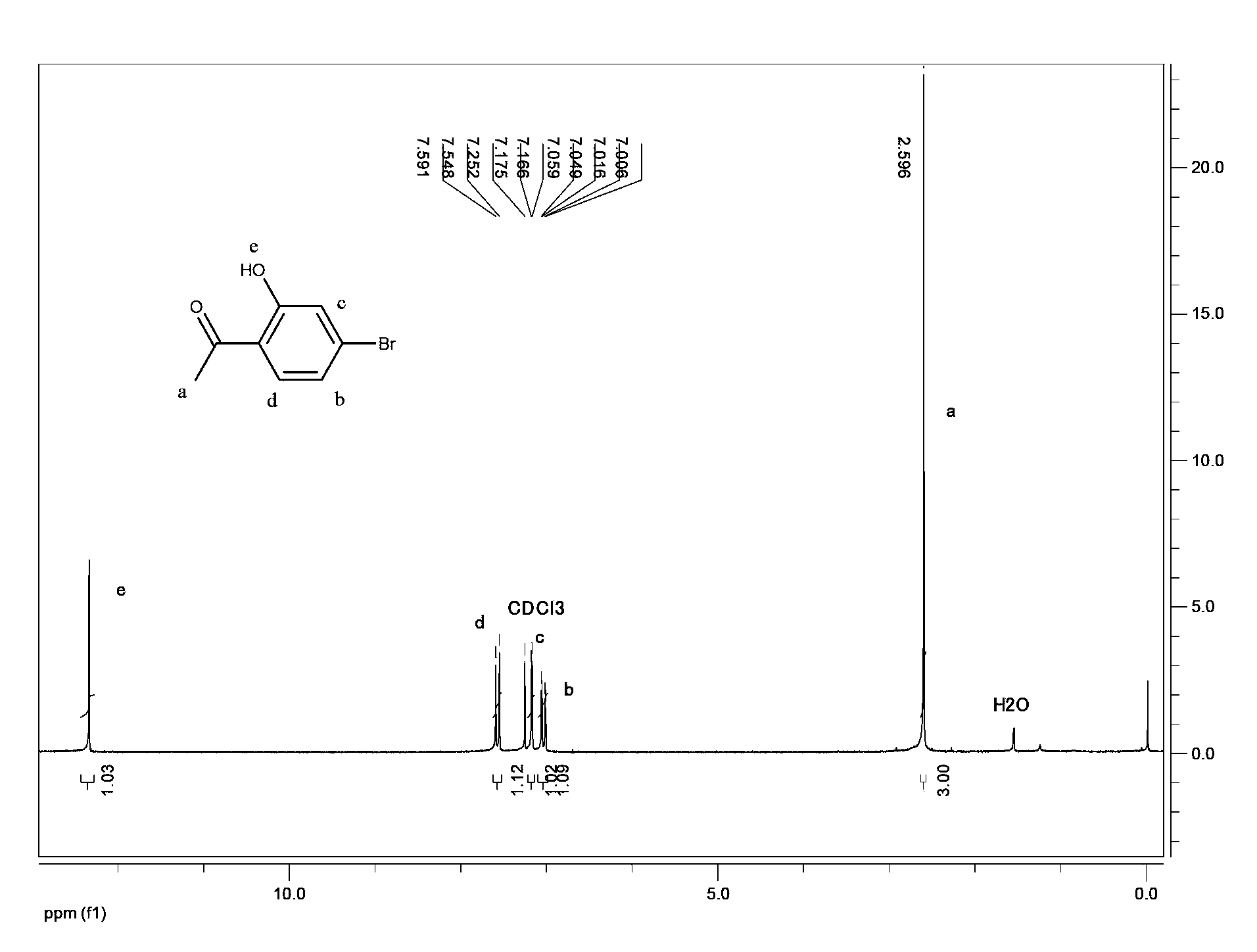
[0070] Preparation of 2-hydroxy-4-bromoacetophenone
[0071]
[0072] (1) Preparation of [sulfuric acid][triethylamine 4-butylammonium sulfonate][zinc oxide] ionic liquid
[0073] Under stirring conditions, 9.4g of 1,4-butane sultone was slowly added dropwise to 7.0g of triethylamine, reacted at 80°C for 2h, cooled to 25°C, filtered with suction, and the filter cake was washed 3 times with ethanol. Dry under vacuum to obtain triethylamine butane sulfonate inner salt.
[0074] Under an ice bath, mix the 15.8 g of triethylamine butane sulfonate in the deionized water solution obtained in the previous step with 6.5 g of sulfuric acid, react at 50°C for 3 hours, cool, and dry in vacuo to obtain [sulfuric acid][triethylamine 4-butylammonium sulfonate] intermediate of ionic liquid.
[0075] Under stirring conditions, add 5.2 g of zinc oxide and 21.3 g of the [sulfuric acid] [triethylamine 4-butylammonium sulfonate] ionic liquid intermediate obtained in the previous step into deionized wate...
Embodiment 2
[0083] Preparation of 2-hydroxy-4-methoxypropiophenone
[0084]
[0085] (1) Preparation of [sulfuric acid][dipropylamine 4-butylammonium sulfonate][copper oxide] ionic liquid
[0086] Under stirring conditions, 21.7g of 1,4-butane sultone was slowly added dropwise to 16.1g of dipropylamine, reacted at 70°C for 2h, cooled to 25°C, filtered with suction, and the filter cake was washed 3 times with ethanol and vacuum After drying, the inner salt of dipropylamine butane sulfonate is obtained.
[0087] Under an ice bath, mix the 36.7g deionized aqueous solution of dipropylamine butane sulfonate obtained in the previous step with 15.2g sulfuric acid, react at 60°C for 5h, cool, and dry in vacuo to obtain [sulfuric acid][dipropylamine 4- Butylammonium sulfonate] Intermediate of ionic liquid.
[0088] Under stirring conditions, add 11.8 g of copper oxide and 49.7 g of the [sulfuric acid] [dipropylamine 4-butylammonium sulfonate] ionic liquid intermediate obtained in the previous step into d...
Embodiment 3
[0096] Preparation of 2-hydroxy-4-methylphenylbenzophenone
[0097]
[0098] (1) Preparation of [sulfuric acid][dipropylamine 4-butylammonium sulfonate][copper oxide] ionic liquid
[0099] Under stirring conditions, 14.1g of 1,4-butane sultone was slowly added dropwise to 10.4g of dipropylamine, reacted at 70°C for 2h, cooled to 25°C, filtered with suction, and the filter cake was washed 3 times with ethanol and vacuum After drying, the inner salt of dipropylamine butane sulfonate is obtained.
[0100] Under an ice bath, mix 23.8g of dipropylamine butane sulfonate in the deionized water solution obtained in the previous step with 9.8g of sulfuric acid, react at 60°C for 5h, cool, and dry in vacuo to obtain [sulfuric acid][dipropylamine 4- Butylammonium sulfonate] Intermediate of ionic liquid.
[0101] Under stirring conditions, add 7.2 g of copper oxide and 32.2 g of the [sulfuric acid] [dipropylamine 4-butylammonium sulfonate] ionic liquid intermediate obtained in the previous step ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com