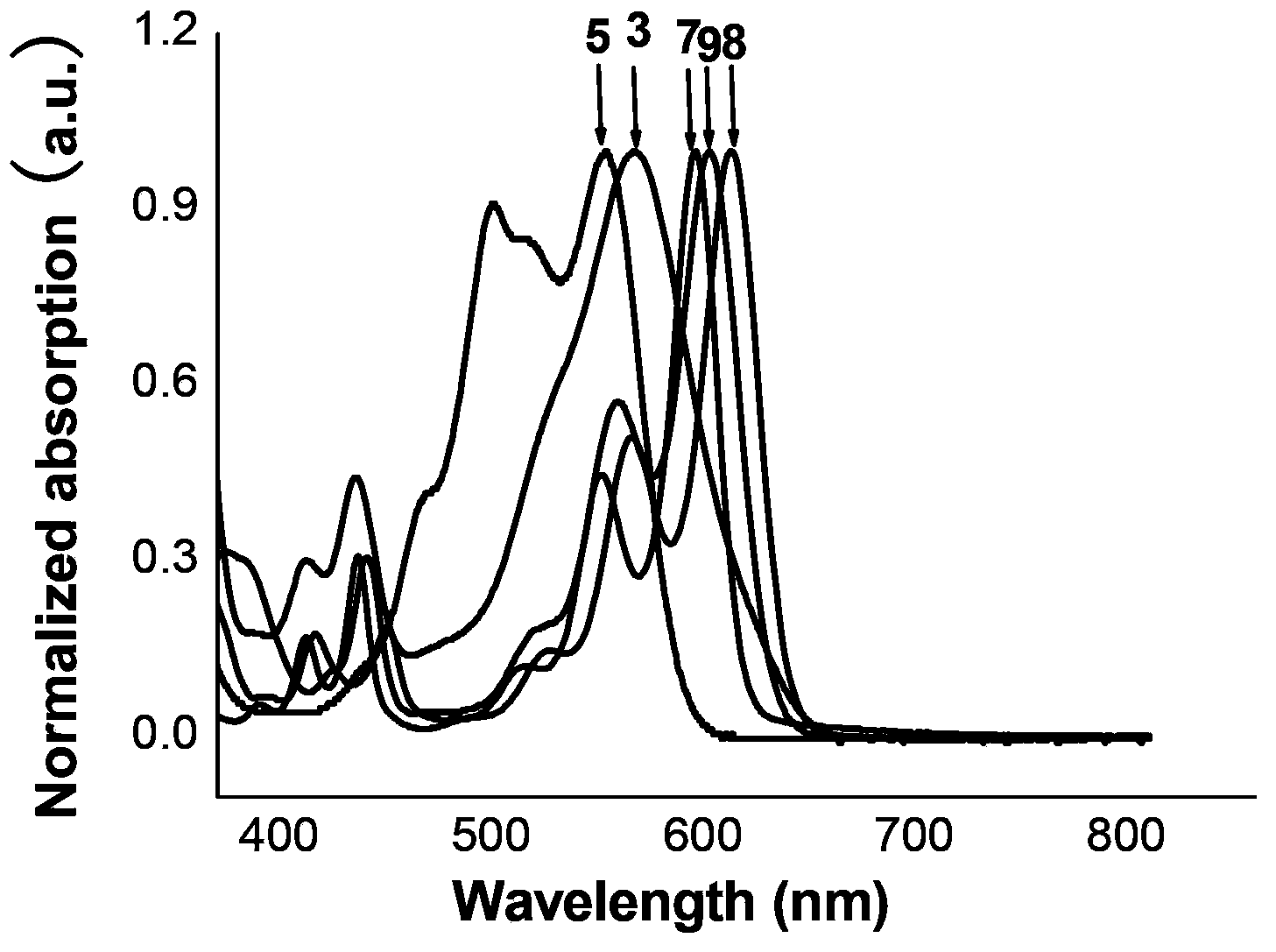
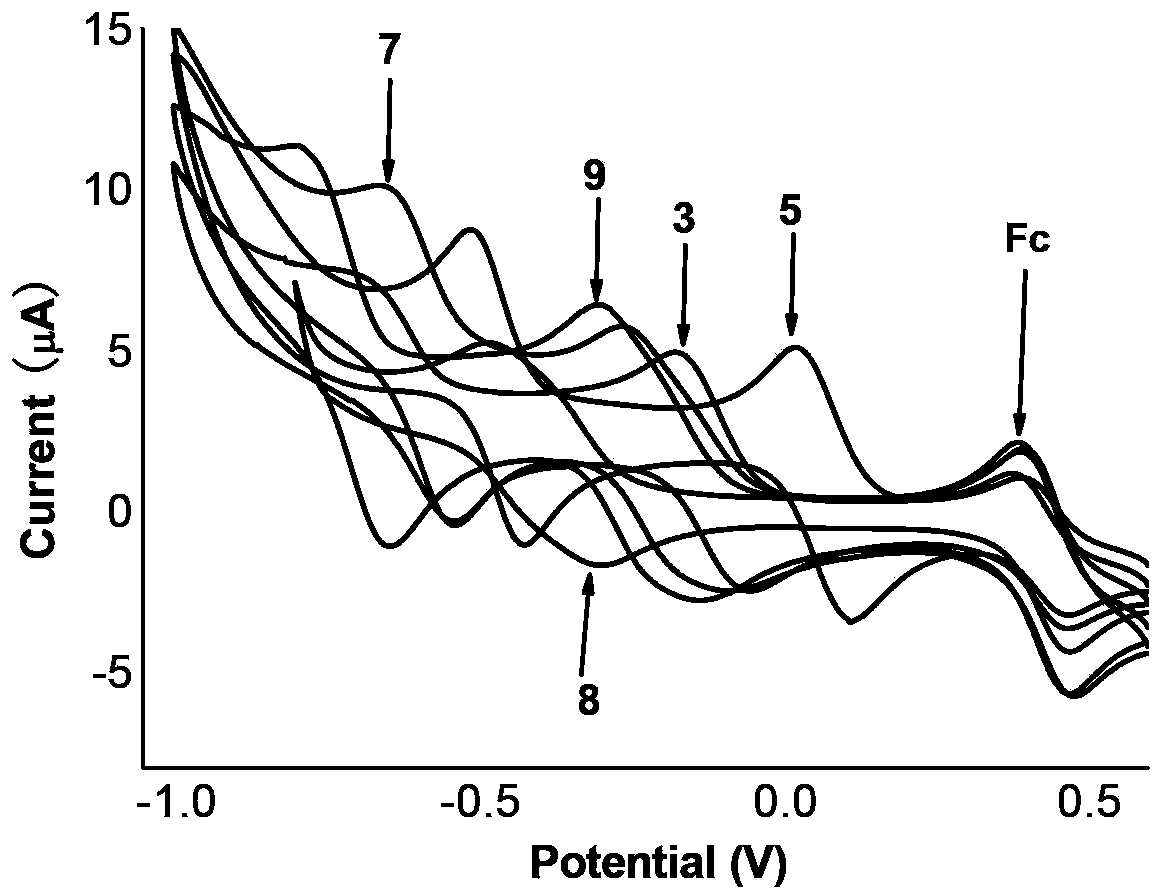
Naphthalimide derivant containing 1, 3-dithiol-2-yliden conjugation unit, preparation method and application
A technology of naphthalene diimide and derivatives is applied in the field of naphthalene diimide organic semiconductor materials, which can solve the problems of restricting the development of flexible electronic functional devices and shortage of n-type organic semiconductor materials with electron mobility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: N-(2-octyl-dodecyl)-N′-(4-tert-butylbenzyl)-[2,3-d:6,7-d′]-bis[2- Synthesis of (1,3-dithio-2-ylidene)-2-propanedicyano]-naphthalene-1,4,5,8-tetracarboxylic acid diimide (1).
[0060] Concrete synthetic steps are:
[0061] Under nitrogen protection, 2,3,6,7-tetrabromonaphthalene tetracarboxylic dianhydride (TBNDA) (300mg, 0.51mmol) and 1,1-dicyanoethylene-2,2-dithiolate sodium (288mg , 1.54mmol) was added to 50mL DMF and heated to 50°C. After stirring for 1 h, 2-octyl-dodecylamine (228 mg, 0.77 mmol) and 4-tert-butylbenzylamine (125 mg, 0.77 mmol) were added, and the reaction was continued at 50° C. for 5 h. Cool to room temperature, pour the reaction solution into 100mL saturated ammonium chloride solution, filter, wash the filter residue with water, dry in vacuo, use dichloromethane / petroleum ether (2:1) as eluent, and use a silica gel column to analyze the crude The product was separated and purified to obtain 50 mg of compound 1 as a brown-red solid wit...
Embodiment 2
[0064] Example 2: N-(2-octyl-dodecyl)-N′-(2,2-diphenyl-ethyl)-[2,3-d:6,7-d′]-bis Synthesis of [2-(1,3-dithio-2-ylidene)-2-propanedicyano]-naphthalene-1,4,5,8-tetracarboxylic diimide (2).
[0065] Concrete synthetic steps are:
[0066] Using 2,2-diphenylethylamine instead of p-tert-butylbenzylamine, the synthesis method was the same as that in Example 1, and a red solid (2) was prepared with a yield of 7%.
[0067] Mass Spectrum: [MS(TOF)]m / z:1003.3(M + ); H NMR spectrum: 1 H-NMR (300MHz, CDCl 3 )δ(ppm):0.839–0.891(m,6H,–CH 3 ),1.236–1.375(m,32H,–CH 2 –), 1.932-2.015 (b, 1H, –CH-), 4.191–4.216 (d, 2H, –CH 2 –),4.780–4.834(t,1H,–CH–),4.952-4.981(d,2H,–CH 2 –), 7.162–7.372 (m, 10H, Ph-H), 7.542-7.569 (d, 2H, Ph-H); elemental analysis calculated value (Anal.Calcd.For) C 56 h 54 N 6 o 4 S 4 :C,67.04;H,5.42;N,8.38;Found:C,67.32;H,5.64;N,8.51.
[0068]
Embodiment 3
[0069] Example 3: N,N'-bis(2-octyl-dodecyl)-[2,3-d]-[2-(1,3-dithio-2-ylidene)-2-propane Dicyano]-[6,7-d′][1,4-dithiacyclohexene-2,3-dinitrile]-naphthalene-1,4,5,8-tetracarboxylic diimide (3 )Synthesis.
[0070] Concrete synthetic steps are:
[0071] Under nitrogen protection, N,N'-bis(2-octyl-dodecyl)-2,3,6,7-tetrabromonaphthalene diimide (TBNDI-C20) (500mg, 0.44mmol) , 1,1-dicyanoethylene-2,2-sodium dithiolate (122mg, 0.66mmol) and 1,2-dicyanoethylene-1,2-sodium dithiolate (150mg, 0.79mmol) were added to In 100mL THF, stirred and reacted at -10°C for 2.5h, cooled to room temperature, poured the reaction solution into 200mL water, dissolved 4×100mL dichloromethane solution for extraction, combined the organic phases, dried, and spun off the solvent under reduced pressure. The crude product was purified by silica gel chromatography (eluent: dichloromethane / petroleum ether, V / V=1 / 1) to obtain 182 mg of compound 3 as a red solid with a yield of 38%.
[0072] Mass Spectrum: MS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electron mobility | aaaaa | aaaaa |
| Electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com