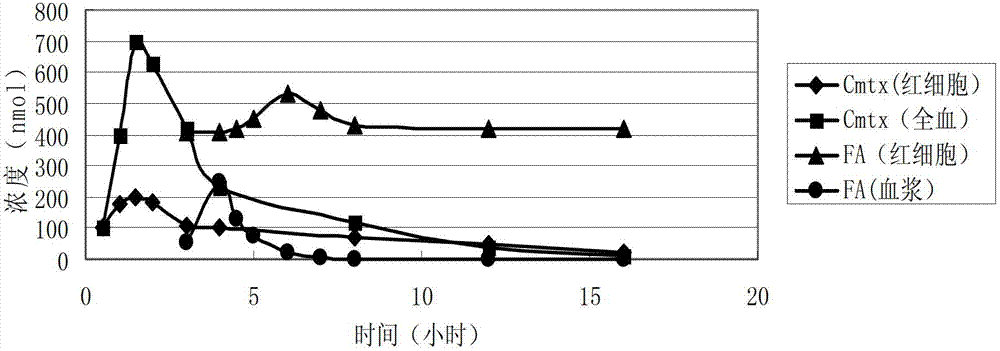
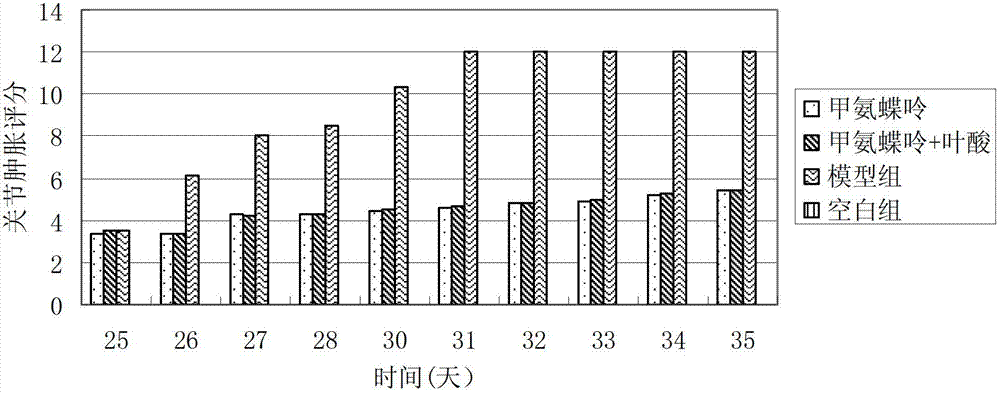
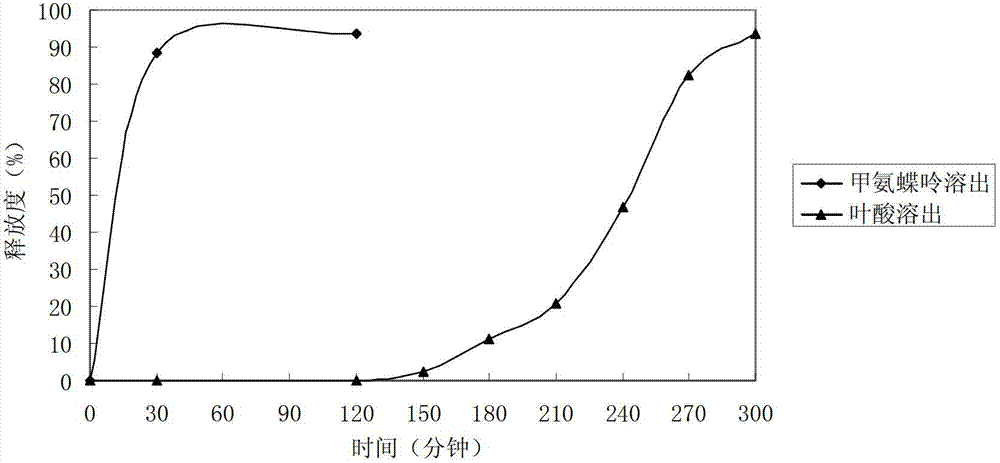
Methionine folic acid compound preparation and its preparation method and use
A compound preparation, the technology of methamphetamine folic acid, which is applied in anti-inflammatory agents, pill delivery, non-central analgesics, etc., can solve the problems of poor compliance, missed doses, and easy to take by mistake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] Both the methotrexate part and the folic acid part in the methotrexate compound preparation can be made into medicinal pellets. The preparation method of pharmaceutical pellets mainly includes two steps: drug loading and coating. Drug loading is to directly coat the drug micropowder on the surface of the round core particle (also known as drug application) or use different techniques to mix the drug and excipients and directly make round pellets. The methods of drug loading of the present invention mainly include: 1. the method of adding medicine to the core of the pill, and 2. the method of directly forming a pill.
[0074] In the present invention, when adopting the drug-on-ball method to prepare methotrexate pellets and folic acid pellets, the methotrexate pellets include blank pellet cores, drug layers and protective layers sequentially from inside to outside; It includes a blank pellet core, a drug layer, an isolation layer and a functional layer in turn. The bla...
Embodiment 1
[0087] 1.1 Prescription of methotrexate pellets (10000 capsules)
[0088] (0.9-1.12mm) sucrose core 1160.5g
[0089] Methotrexate 36.2g
[0090] PVP S630 27.5g
[0091] HPMC 24.9g
[0092] Water 2049ml
[0093] Preparation process: add 27.5g of PVP S630 and 36.2g of methotrexate to 1500ml of water to make the upper drug solution; add 24.9g of HPMC to 549ml of water to make the protective layer liquid. 1160.5g average particle diameter is 0.9-1.12mm sucrose blank pellet core is placed in the fluidized bed and fluidized for 10 minutes, sprayed with liquid medicine, protective layer liquid successively, obtains methotrexate pellets (medicine liquid and protective layer The liquid is continuously stirred during the whole drug application and coating process). This process adopts the fluidized bed bottom spraying process.
[0094] 1.2 Prescription of folic acid pellets (10000 capsules)
[0095] (0.9-1.12mm) sucrose core 105.3g
[0096] Folic acid 9.1g
[0097] PVP S630 1.8...
Embodiment 2
[0108] 2.1 Prescription of methotrexate pellets (10000 capsules)
[0109] 0.8-1.0mm starch ball core 99.6g
[0110] Methotrexate 21.8g
[0111] PVP VA64 6.8g
[0112] HPC 14.2g
[0113] water 85ml
[0114] Preparation process: add 6.8g of PVP VA640 and 21.8g of methotrexate to 40ml of water to make the upper drug solution; add 14.2g of HPC to 45ml of water to make the protective layer liquid. Put 99.6g of starch blank cores with an average particle size of 0.8-1.0mm in a fluidized bed for 10 minutes, spray the drug solution and the protective layer liquid successively to obtain the methotrexate pellets (the drug solution and the protective layer The liquid is continuously stirred during the whole drug application and coating process). This process adopts the fluidized bed bottom spraying process.
[0115] Same as Example 1.1
[0116] 2.2 Prescription of folic acid pellets (10000 capsules)
[0117] 0.6-0.8mm starch ball core 607.5g
[0118] Folic acid 21.8g
[0119] P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com