Synthetic method of 1, 3-dioxane type organic compounds
A technology of dioxane, organic compound, applied in 1 field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
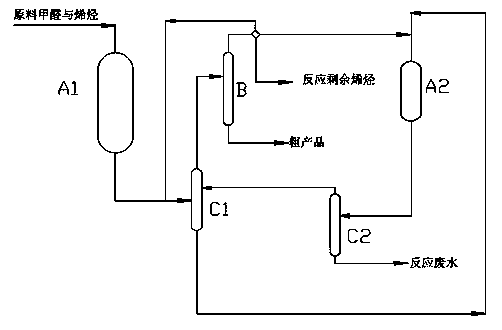
[0023] Example 1: (a) Use deionized 37wt% industrial formaldehyde and butane containing 50wt% 1-butene as raw materials, and send them into the first-stage catalytic fixed-bed reactor A1 at a molar ratio of 1.8:1, and the reaction temperature is 100 ℃, the reaction pressure is 2MPa, and the reaction is catalyzed by macroporous strongly acidic polystyrene cation exchange resin;
[0024] (b) The reaction liquid extracted from the first-stage catalytic fixed-bed reactor A1 is sent to the first-stage extraction and separation tower C1, and the extracted oil phase is sent to the separation tower B for separation. The temperature of the tower bottom is 163 ° C, and the temperature of the top of the tower is 48 ° C. The pressure is 0.45MPa; the extracted aqueous phase is sent to the second-stage catalytic fixed-bed reactor A2, the reaction temperature is 105°C, and the reaction pressure is 2MPa, and the reaction is catalyzed by macroporous strongly acidic polystyrene cation exchange...
Embodiment 2
[0028] Example 2 (a) With deionized 37wt% industrial formaldehyde and butane containing 50wt% 2-butene as raw material, send it into the first-stage catalytic fixed-bed reactor A1 at a molar ratio of 1.8:1, and the reaction temperature is 100°C, the reaction pressure is 2MPa, and the reaction is catalyzed by macroporous strongly acidic polystyrene cation exchange resin;
[0029] (b) The reaction liquid extracted from the first-stage catalytic fixed-bed reactor A1 is sent to the first-stage extraction and separation tower C1, and the mixed material that is left over from the reaction and separated and replenished by the separation tower B is used for extraction and separation, and the resulting oil phase is extracted Sent to the separation tower B for separation, the temperature of the tower kettle is 163°C, the temperature of the tower top is 48°C, and the pressure is 0.45MPa; the extracted aqueous phase is sent to the second-stage catalytic fixed-bed reactor A2, the reaction...
Embodiment 3
[0033] Example 3 : : (a) Use deionized 37wt% industrial formaldehyde, butane containing 25wt% 1-butene and 25wt% 2-butene as raw materials, and send them into the first-stage catalytic fixed-bed reactor at a molar ratio of 1.8:1 A1, the reaction temperature is 100°C, the reaction pressure is 2MPa, and the reaction is catalyzed by macroporous strongly acidic polystyrene cation exchange resin;
[0034] (b) The reaction liquid extracted from the first-stage catalytic fixed-bed reactor A1 is sent to the first-stage extraction and separation tower C1, and the mixed material that is left over from the reaction and separated and replenished by the separation tower B is used for extraction and separation, and the resulting oil phase is extracted Sent to the separation tower B for separation, the temperature of the tower kettle is 163°C, the temperature of the tower top is 48°C, and the pressure is 0.45MPa; the extracted aqueous phase is sent to the second-stage catalytic fixed-bed r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com