Synthesis and application of fluorescent probe capable of reversibly detecting hypoxic environment
A fluorescent probe and fluorescent group technology, which is used in the synthesis of reversible hypoxia fluorescent probes and in the application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In this example, a cyanine dye is preferably used as the fluorescent group, and the connecting arm is C 2 alkane, a fluorescent probe synthesized by benzoquinone redox acceptor group, the fluorescent group can also be rhodamine derivatives, fluorescein and its derivatives, fluoroboryrrole derivatives and acenaphthylenine derivatives any kind.
[0029] The synthetic formula of the compound cyanine dye-benzoquinone (Cy7-BQ):
[0030]
[0031] 1) Synthesis of 2,5-dimethoxy-3,4,6,-trimethylphenylacrylic acid using Wittig reagent:
[0032] Add ethyl bromoacetate (1mmol), tributylphosphine (1mmol) and dichloromethane (30mL) sequentially into a 50mL single-necked flask and stir at room temperature to react. After about 4h, a white solid was filtered by suction. Put the obtained solid compound into another 50mL single-necked flask, add anhydrous THF (20mL), potassium tert-butoxide (1.1mmol), react at room temperature for about 3h, and then add 3,4,6-trimethyl- 2,5-Dimethoxy...
Embodiment 2
[0054] In this example, cyanine dyes are preferably used as fluorescent probes synthesized by fluorescent groups and benzoquinone redox acceptors.
[0055] The synthetic formula of the compound cyanine dye-(3,4,6-trimethyl-2,4-benzoquinoyl)-3-methyl-1-butane (Cy7-TBQ):
[0056]
[0057] 1) Synthesis of 6-hydroxy-4,4,7,8-tetramethylchromen-2-one:
[0058] 2,3,5-trimethyl-1,4-diphenol as raw material and 3-methyl-butenoic acid methyl ester are reacted at a molar ratio of 1:1 to obtain 6-hydroxy-4,4,7,8 -Tetramethylchromen-2-one.
[0059] 2) Synthesis of intermediate (3,4,6-trimethyl-2,4-benzoquinone)-3-methyl-butyric acid:
[0060] The intermediate (3,4,6-trimethyl -2,4-benzoquinoyl)-3-methyl-butanoic acid.
[0061] 3) Synthesis of (3,4,6-trimethyl-2,4-benzoquinoyl)-3-methyl-1-butanoic acid:
[0062] Reduction of (3,4,6-trimethyl-2,4-benzoquinone)-3-methyl-butanoic acid compound using lithium aluminum hydride as a reducing agent afforded (3,4,6-trimethyl- 2,4-Benzoquino...
Embodiment 3
[0066] Probe pair redox system determination:
[0067] Hydroquinone reductase (lyophilized powder) was purchased from Sigma-Aldrich. All tests were done with 40 µL microfluometry cuvettes.
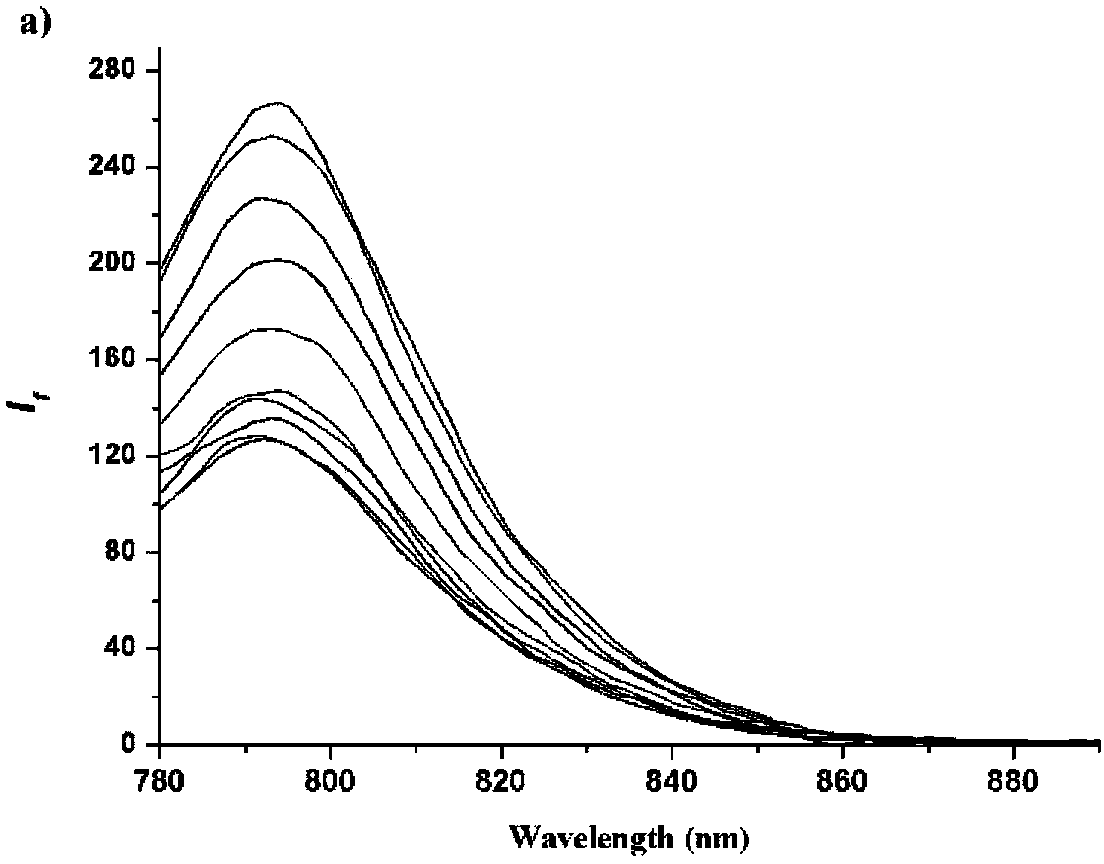
[0068] figure 1 It is a schematic diagram of the fluorescence spectrum of the probe catalyzed by hydroquinone reductase in Tris-HCl (containing 1% DMSO) buffer at pH=7.4.
[0069] Such as figure 1 As shown, the DMSO master solution of the prepared compound Cy7-TBQ / Cy7-BQ fluorescent probe was added to the test buffer (0.01M NADH, PBS buffer, pH7.0), and the final DMSO concentration was determined to be 1%. Firstly, the hydroquinone reductase was diluted to a certain concentration, so that the final concentration in the test system was maintained at 10 μg / mL. The temperature during the test was kept at 37°C, the excitation wavelength of the fluorescence was 790nm, the fluorescence response curve was collected every 30s, and the spectral signal near 790nm was collected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com