Clindamycin hydrochloride preparation for injection and preparation method thereof
A technology for clindamycin hydrochloride and injections, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve the problem of no relative drug advantage and inability to effectively control drug release Speed, adverse drug reactions and other issues, to achieve the effect of reducing the occurrence of adverse reactions, improving bioavailability, and maintaining pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
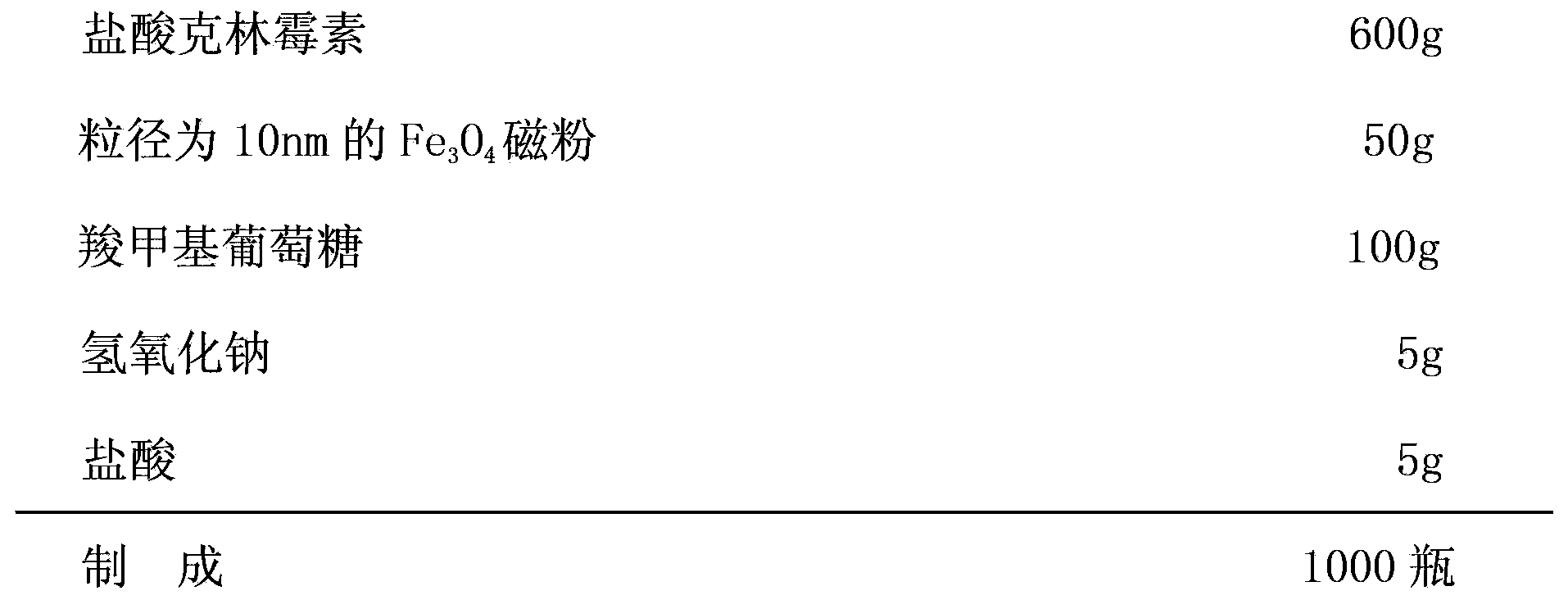
[0019] formula:
[0020]
[0021] Preparation:
[0022] ①Dissolve clindamycin hydrochloride in 2000ml of water for injection, and stir at room temperature of 25°C until clear to obtain solution A;
[0023] ② Sodium hydroxide and hydrochloric acid are configured into a clear solution with a concentration of 10mol / L, and the pH value is adjusted to 5.8 to obtain solution B;
[0024] ③ Fe 3 o 4 Mix the magnetic powder and carboxymethyl glucose, add liquid paraffin covering the mixture, add one-third of the amount of glutaraldehyde to the magnetic powder and react for 30 minutes while stirring at room temperature at 40°C, adjust the pH value of the solution to 9, continue the reaction for 30 minutes, and cool , washed and dried to obtain coated nano-Fe 3 o 4 Particle carboxymethylglucose magnetic microspheres C;
[0025] ④ Mix solution A and solution B evenly, add coated nano-Fe 3 o 4 Particle carboxymethylglucose magnetic microspheres C were stirred at room temperature...
Embodiment 2
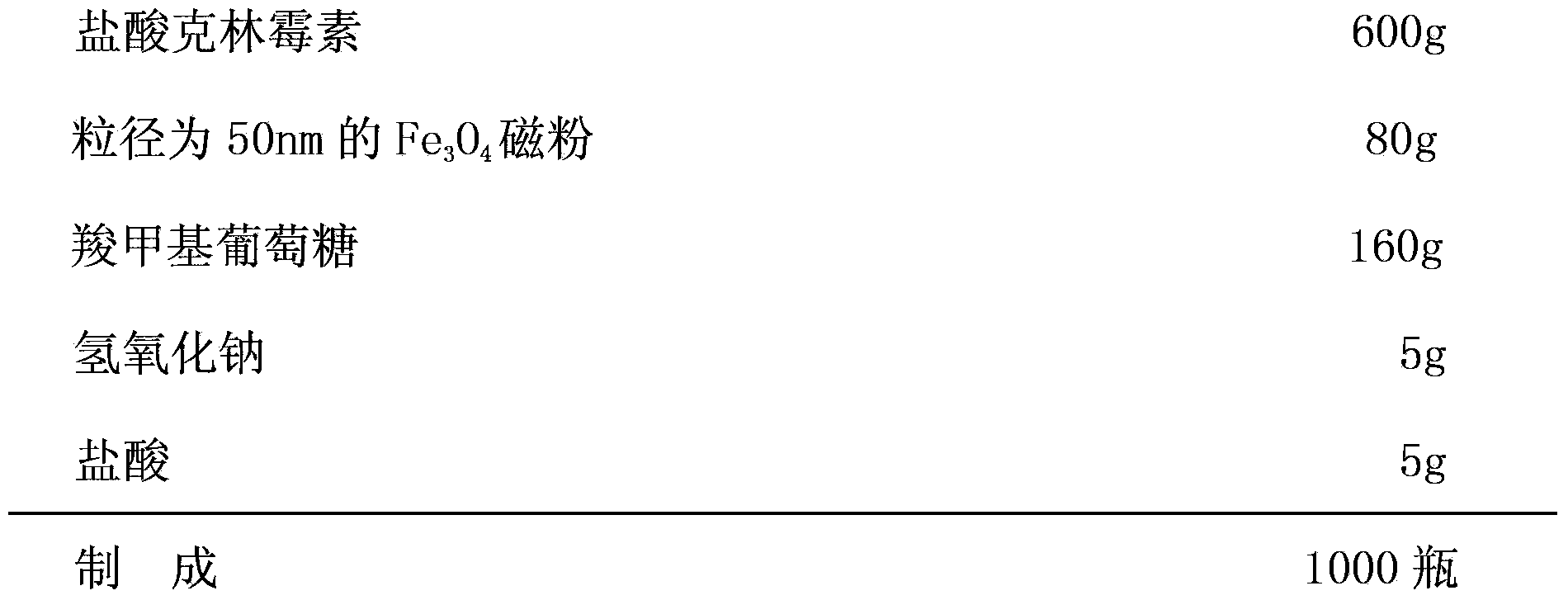
[0028] formula:
[0029]
[0030] The preparation method is the same as in Example 1.
Embodiment 3
[0032] formula:
[0033]
[0034]
[0035] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com