Chiral organic small molecular compound of S-prolinol having cyclopropane structure and synthetic method of chiral organic small molecular compound
A small molecular compound, prolinol technology, applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problem of high reagent cost, harsh process conditions, enantiomer Low structural selectivity and other issues, to achieve the effect of simple operation, reasonable process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
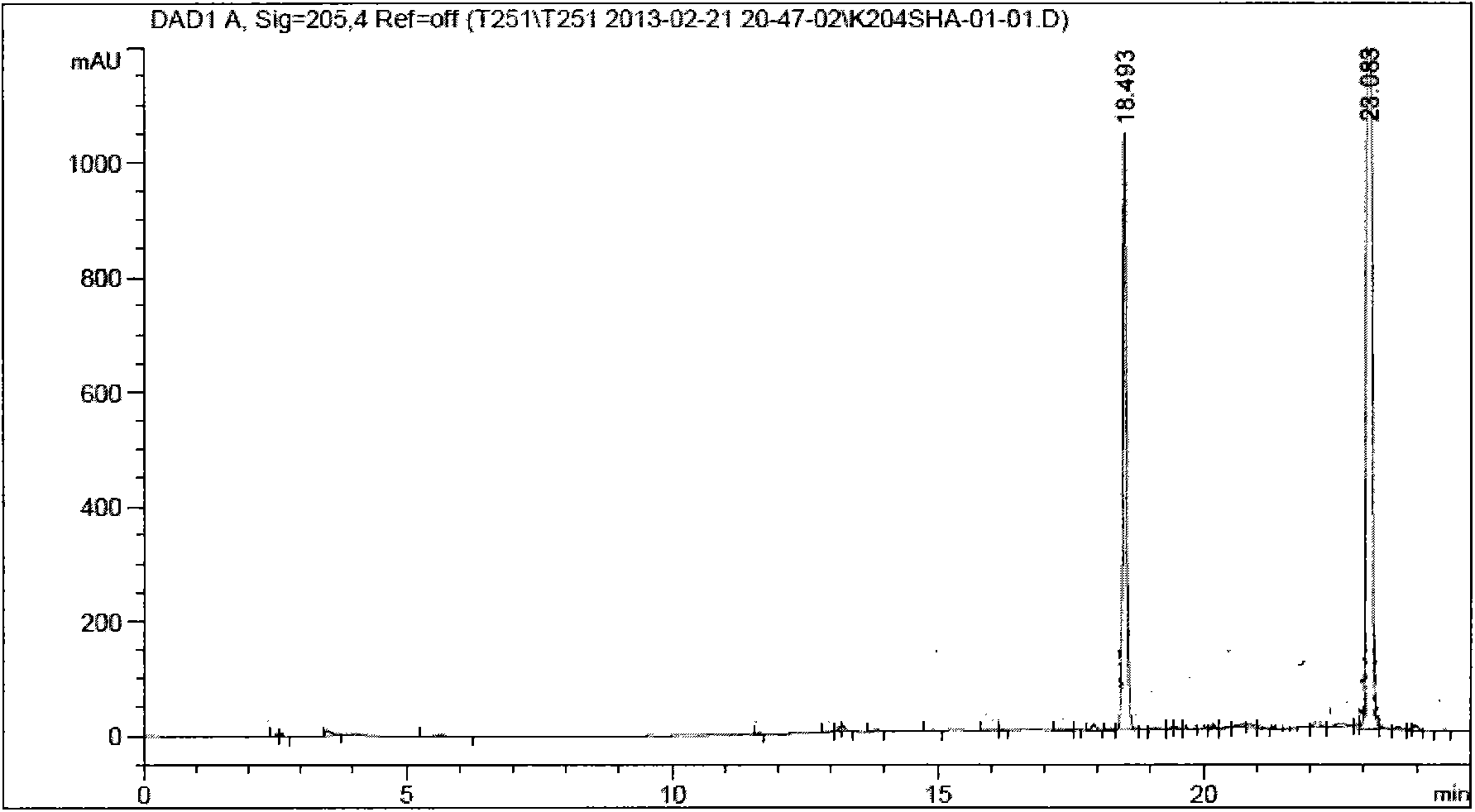
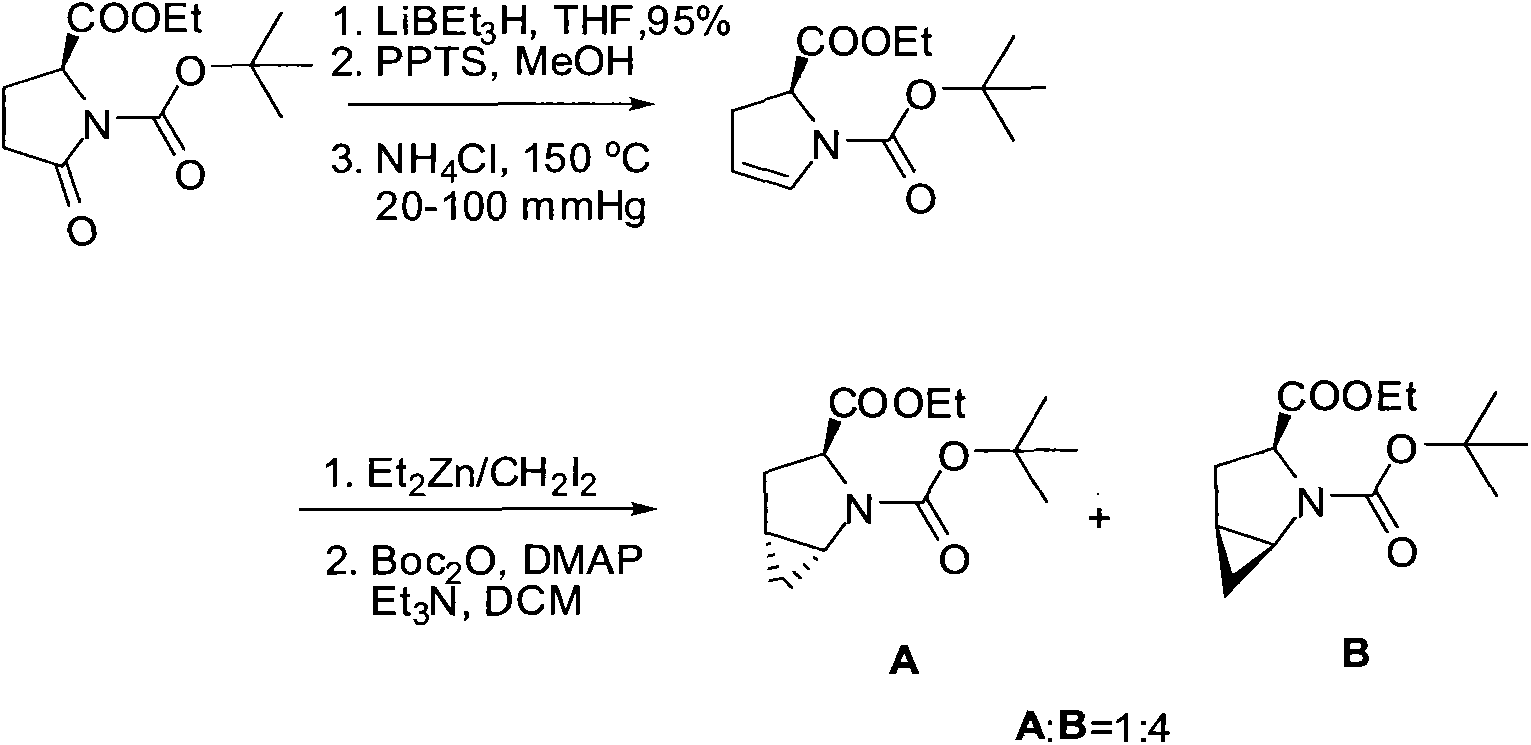
[0055] (1S, 3S, 5S)-N-tert-butoxycarbonyl-2-azabicyclo[3,1,0]hexane-3-ethyl carboxylate 1 and (1R, 3S, 5R)-N-tert-butoxy Preparation of ethyl carbonyl-2-azabicyclo[3,1,0]hexane-3-carboxylate 2
[0056] in N 2 Under protection, (S)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrole carboxylic acid ethyl ester (provided by Shanghai Tongchang Biomedical Technology Co., Ltd., CAS) was added to a dry 250ml two-necked reaction flask No.: 178172-26-4) (34.0g, 0.13mol) was dissolved in 200ml of dichloromethane (DCM), cooled to -15°C, and diethylzinc (Et 2 Zn) (1.2eq, 0.17mol), stirring reaction 0.5h after completion of dropwise addition, dropwise addition of chloroiodomethane (CH 2ClI) (1.1eq, 0.15mol) in dichloromethane (DCM) solution, insulated at -15°C and stirred for 24h, after the reaction was completed, the reaction was quenched with an aqueous solution of 13% ethylenediaminetetraacetic acid (EDTA) with a concentration of 13% by mass , heated to 25°C, stirred for 2 hours, stood st...
Embodiment 2
[0065] Preparation of (1S, 3S, 5S)-N-tert-butoxycarbonyl-2-azabicyclo[3,1,0]hexane-3-methanol 3
[0066] The three-necked reaction flask was added with (1S, 3S, 5S)-N-tert-butoxycarbonyl-2-azabicyclo[3,1,0]hexane-3-carboxylate ethyl ester prepared in Example 1, namely compound 1 ( 25.5g, 0.10mol), add anhydrous tetrahydrofuran to dissolve, control the temperature at 20°C, add lithium aluminum tetrahydrogen (LiAlH 4 ), added 3.8 g (0.10 mol) of lithium aluminum tetrahydrogen in total, then kept the temperature at 20° C. and stirred for 4 hours, concentrated tetrahydrofuran under reduced pressure, added 1% dilute hydrochloric acid dropwise after concentration, separated the upper organic layer, and added Add dichloromethane (DCM), then stir for 10-20 minutes, remove the lower organic layer, add dichloromethane (DCM) to the water layer, remove the lower organic layer, combine the organic layers, and wash with an appropriate amount of anhydrous sodium sulfate Dry and continue to ...
Embodiment 3
[0071] Preparation of (1R,3S,5R)-N-tert-butoxycarbonyl-2-azabicyclo[3,1,0]hexane-3-methanol 5
[0072] The three-necked reaction flask was added with (1R, 3S, 5R)-N-tert-butoxycarbonyl-2-azabicyclo[3,1,0]hexane-3-ethyl carboxylate prepared in Example 1, namely compound 2 ( 2.55g, 0.01mol), add anhydrous tetrahydrofuran to dissolve, control the temperature at 20°C, add lithium aluminum tetrahydrogen (LiAlH 4 ), add 0.38g (0.01mol) of lithium tetrahydrogen aluminum, then keep the temperature at 20°C and stir for 4 hours, concentrate the tetrahydrofuran under reduced pressure, add 1% dilute hydrochloric acid dropwise after the concentration, remove the upper organic layer, and refill the water layer Add dichloromethane (DCM), then stir for 10-20 minutes, remove the lower organic layer, add dichloromethane (DCM) to the water layer, remove the lower organic layer, combine the organic layers, and wash with an appropriate amount of anhydrous sodium sulfate Dry and continue to concen...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap