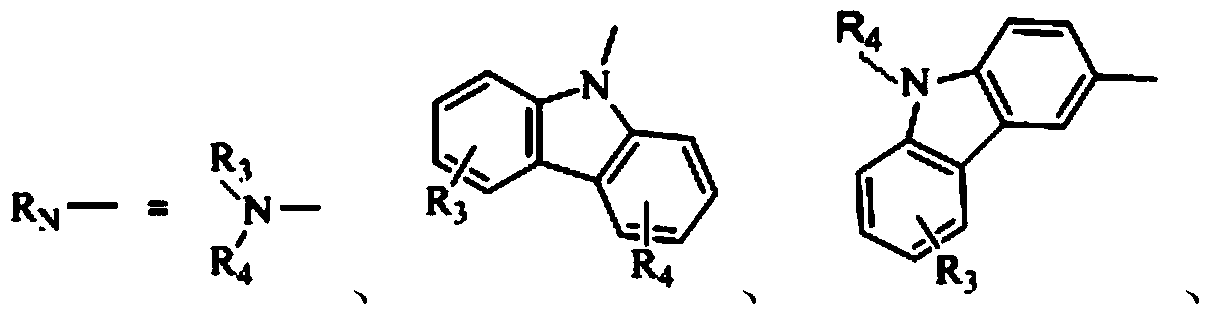
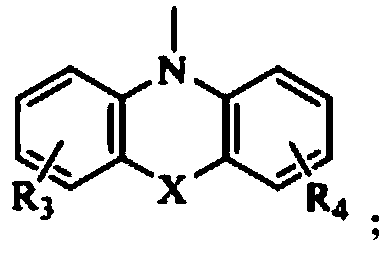
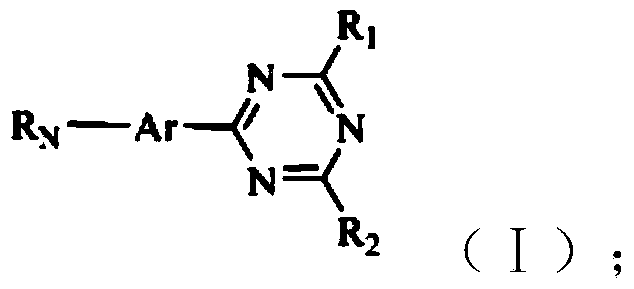
1,3,5-triazine derivative and application thereof in white organic electroluminescent light emitting diode
A technology of triazine derivatives and compounds, applied in the field of 1,3,5-triazine derivatives and its application in white light organic electroluminescent diodes, which can solve problems other than light-emitting mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The synthetic route of compound I1 is as follows:
[0074]
[0075] Synthesis of compound I1: 0.240g 2-chloro-4,6-diphenyl-1,3,5-triazine, 0.287g4-(9H-9-carbazolyl)phenylboronic acid, 0.116g tetrakis(triphenyl) phosphine palladium), 6mL of sodium carbonate solution with a concentration of 2mol / L, 8mL of toluene and 4mL of ethanol (analytical grade) were heated and stirred at 90°C for 12h under nitrogen protection. After the reaction solution was extracted with chloroform, washed with water and dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure to obtain a crude product. The crude product was separated on a silica gel column. The eluent used was a mixture of dichloromethane and petroleum ether at a volume ratio of 1:4. After drying in a vacuum oven, 0.309 g of white solid I1 was obtained with a yield of 65%.
[0076] MS (m / e) of product I1: 475, corresponding to: C33H22N4=475; 1 H NMR (400MHz, CDCl 3 )δ9.03(d,J=8.5Hz,2H),8.82(dd,...
Embodiment 2
[0078] The synthetic route of compound I2 is as follows:
[0079]
[0080] Synthesis of compound I2: 0.900g 2-chloro-4,6-diphenoxy-1,3,5-triazine, 0.867g4-(9H-9-carbazolyl)phenylboronic acid, 0.346g tetrakis(tri Phenylphosphine palladium), 9 mL of sodium carbonate solution with a concentration of 2 mol / L, 12 mL of toluene (analytical pure) and 6 mL of ethanol (analytical pure) were heated and stirred at 45 °C for 10 h under nitrogen protection. After the reaction solution was extracted with chloroform, washed with water and dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure to obtain a crude product. The crude product was separated on a silica gel column, and the eluent used was a mixture of dichloromethane and petroleum ether at a volume ratio of 1:3. After drying in a vacuum oven, 1.214 g of white solid I2 was obtained, with a yield of 80%.
[0081] MS (m / e) of product I2: 506, corresponding to: C33H22N4O2=506; 1 H NMR (400MHz, CDCl ...
Embodiment 3
[0083] The synthetic route of compound I3 is as follows:
[0084]
[0085] Synthesis of compound I3: 0.240g 2-chloro-4,6-diphenyl-1,3,5-triazine, 0.280g 4-triphenylamine borate, 0.116g tetrakis(triphenylphosphine palladium), the concentration is 2mol / 6 mL of sodium carbonate solution in L, 8 mL of toluene (analytical pure) and 4 mL of ethanol (analytical pure) were heated and stirred at 90 ° C for 12 h under nitrogen protection. After the reaction solution was extracted with chloroform, washed with water and dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure to obtain a crude product. The crude product was separated on a silica gel column, and the eluent used was a mixture of dichloromethane and petroleum ether at a volume ratio of 1:4. After drying in a vacuum oven, 0.350 g of light yellow solid I3 was obtained, with a yield of 74%.
[0086] MS (m / e) of product I3: 476, corresponding to: C33H24N4=476; 1 H NMR (400MHz, CDCl 3 )δ8.75(d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum brightness | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com