Spiro quinazoline derivative and preparation method thereof
A technology for quinazoline and derivatives, which is applied in the field of spirocyclic quinazoline derivatives and their preparation, can solve the problems of harsh reaction conditions, low reaction yield, and many by-products, and achieve simple post-processing and high product activity , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of compound a:
[0040]
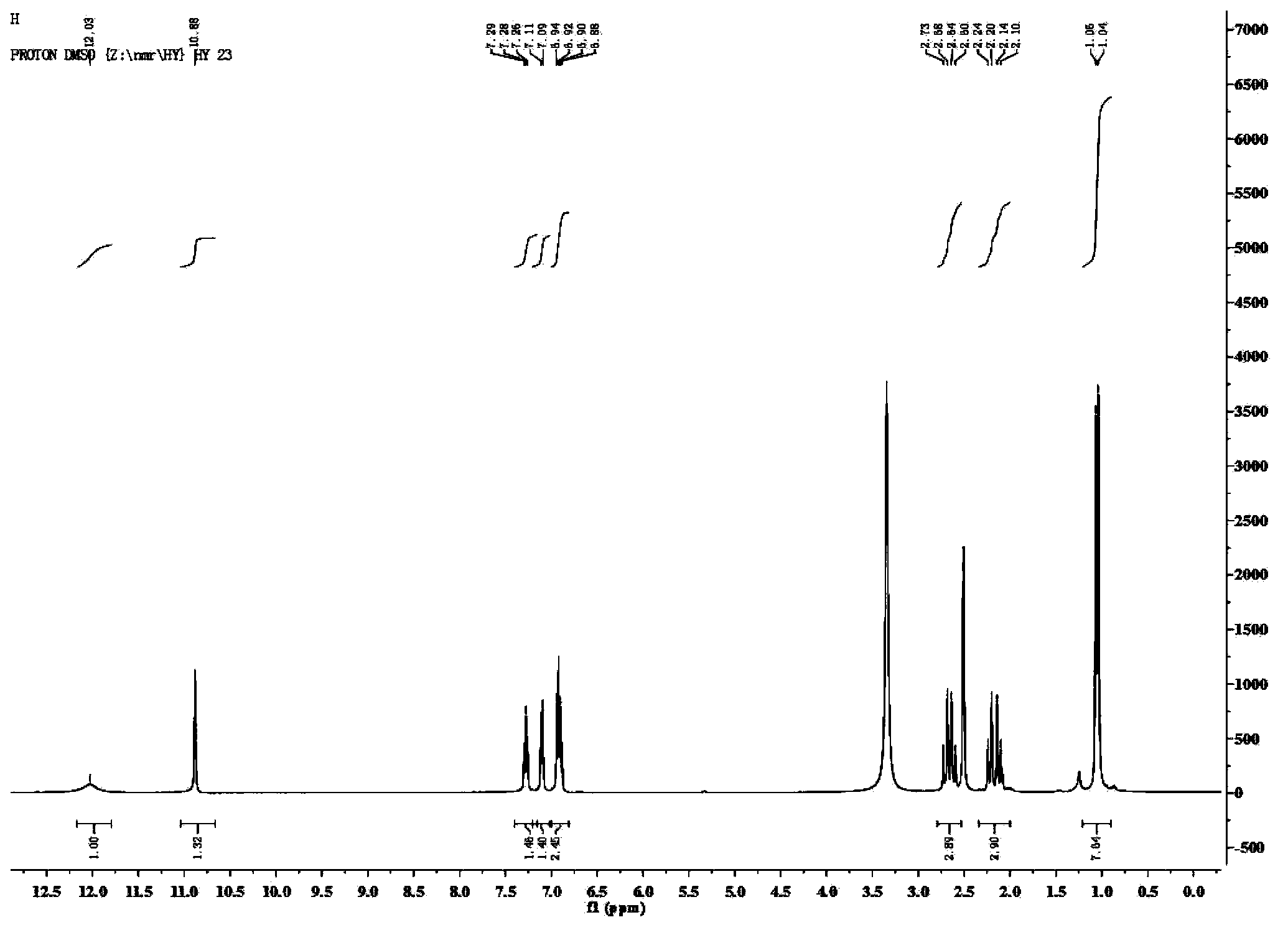
[0041] Add 1mmol of 5-amino-1H-tetrazolium, 1mmol of isatin, 1mmol of 5,5-dimethyl-1,3-cyclohexanedione, 4002mL of polyethylene glycol, 2mL of water and para Toluenesulfonic acid 0.5mmol, warmed up to 80°C, and reacted under stirring for 12h. The reaction solution was cooled to 40-50°C, poured into 30 mL of cold water, and centrifuged to obtain a solid. The solid was recrystallized with 4 mL of acetonitrile, and the recrystallized solid was vacuum-dried at a vacuum degree of 0.1Mpa and a temperature of 50°C to obtain spiro[indoline-3,9'-tetrazole[5,1-b ]quinazoline]-2,8'(5'hydrogen)-one derivatives. Yield: 86%, white solid; mp310-312℃; 1 H NMR (400MHz, DMSO-d 6 )δ12.03(s,1H),10.88(s,1H),7.28(t,J=7.6Hz,1H),7.10(d,J=7.3Hz,1H),6.91(dd,J=15.0,7.6 Hz,2H),2.66(dd,J=35.1,17.1Hz,2H),2.15(dt,J=24.9,12.5Hz,2H),1.05(d,J=10.0Hz,6H); 13 C NMR (101MHz, DMSO-d 6 )δ193.14, 173.55, 153.15, 148.74, 143.02, 130.75, 130.16, 123.99, 122.66...
Embodiment 2
[0043]Preparation of compound b:
[0044]
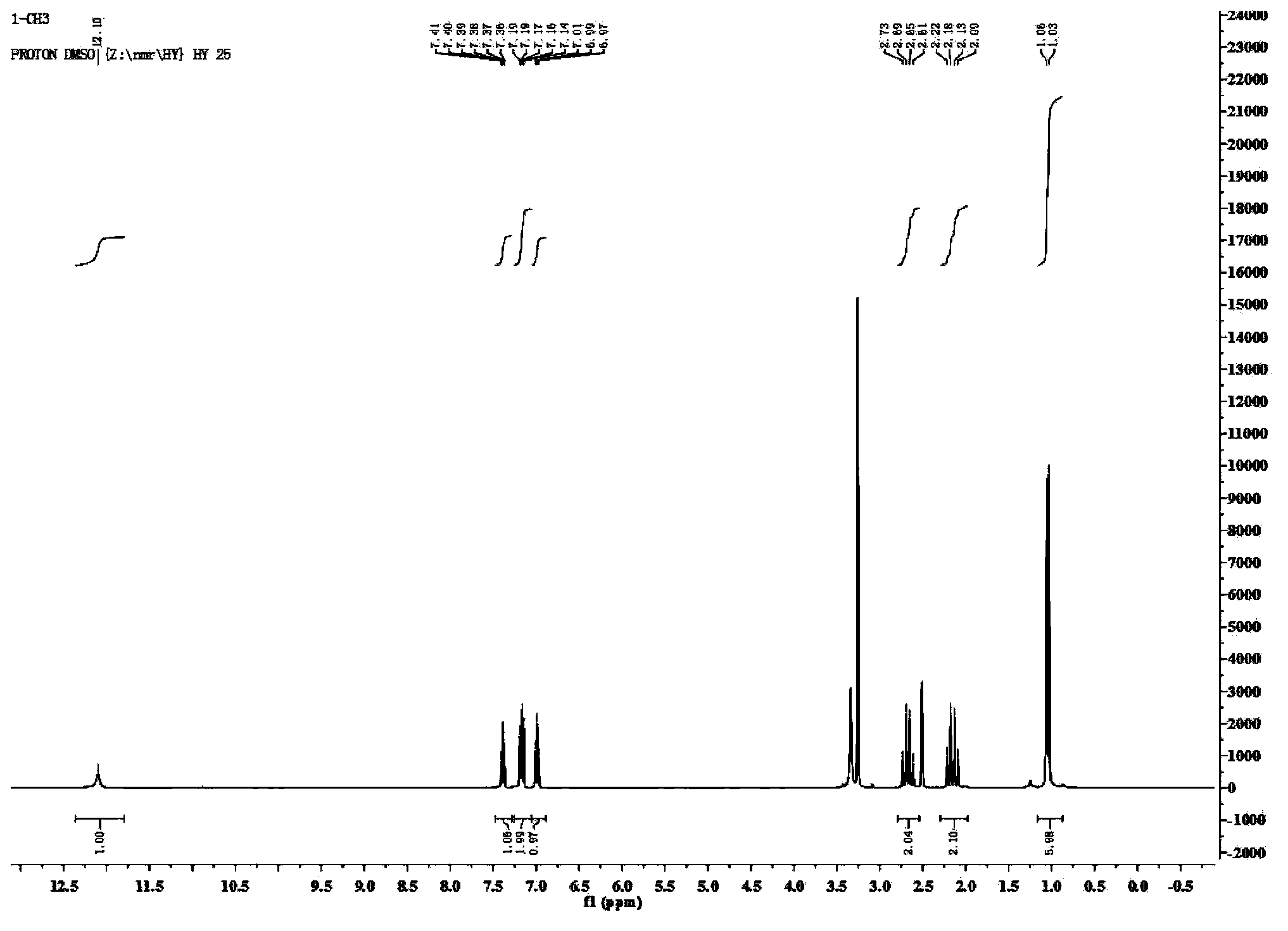
[0045] Add 1mmol of 5-amino-1H-tetrazolium, 1mmol of 5-fluoroisatin, 1mmol of 5,5-dimethyl-1,3-cyclohexanedione, 4004mL of polyethylene glycol and 36 % concentrated hydrochloric acid 0.5mmol, warmed up to 80°C, and reacted under stirring for 12h. The reaction solution was cooled to 40-50°C, poured into 30 mL of cold water, and centrifuged to obtain a solid. The solid was recrystallized with 4 mL of acetonitrile, and the recrystallized solid was vacuum-dried at a vacuum degree of 0.1Mpa and a temperature of 50°C to obtain spiro[indoline-3,9'-tetrazole[5,1-b ]quinazoline]-2,8'(5'hydrogen)-one derivatives. Yield: 90%, white solid; mp308-310℃; 1 H NMR (400MHz, DMSO-d 6 )δ12.08(s,1H),10.92(s,1H),7.21(dd,J=7.9,2.2Hz,1H),7.12(s,1H),6.93(d,J=4.2Hz,1H), 2.65(q,J=17.1Hz,2H),2.18(d,J=10.9Hz,2H),1.05(s,6H); 13 C NMR (101MHz, DMSO-d 6 )δ193.25, 173.68, 159.85, 157.49, 153.47, 148.69, 139.36, 131.44, 117.02, 112.45, 111.36, 104.57, 65.63...
Embodiment 3
[0047] Preparation of compound c:
[0048]
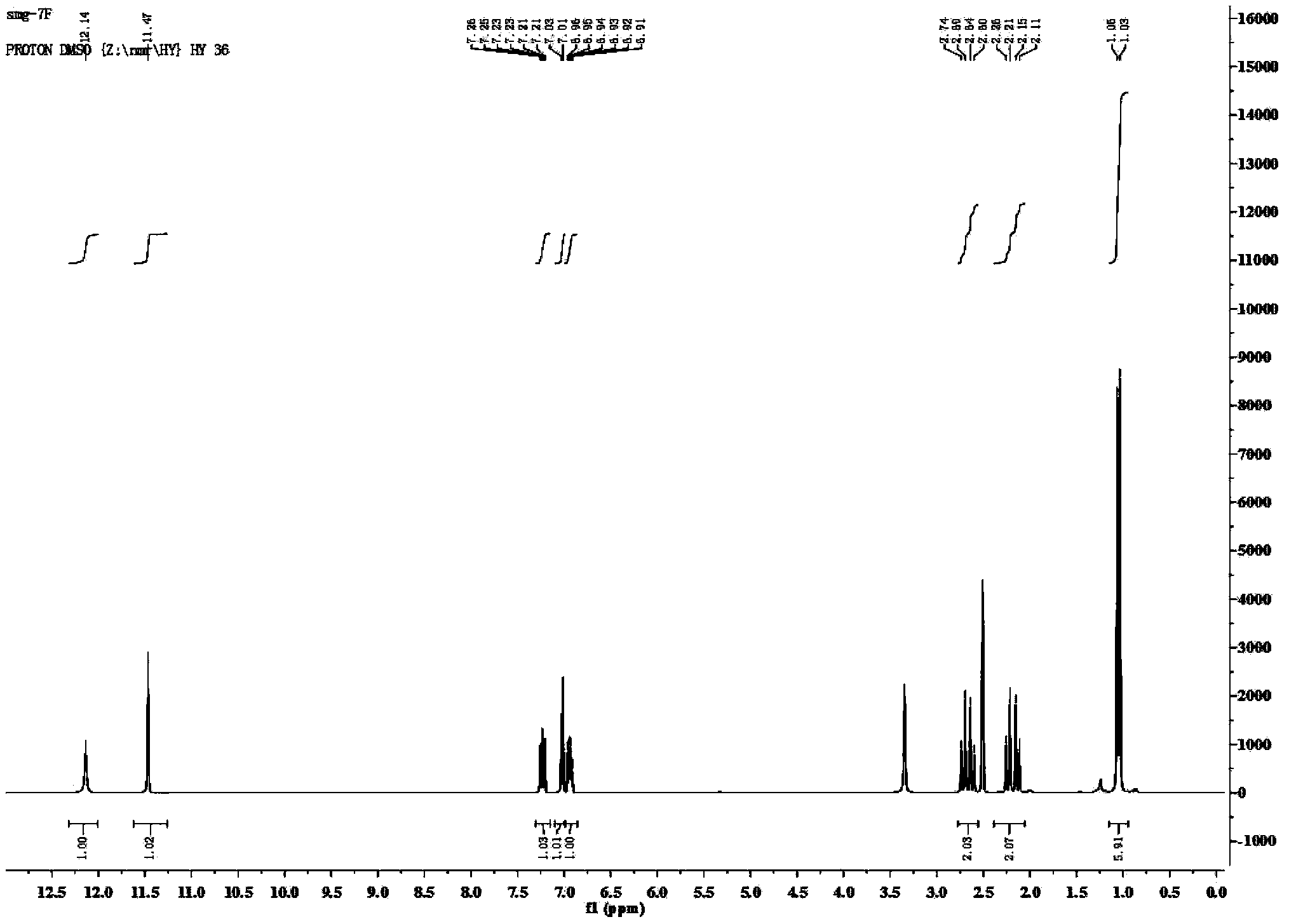
[0049] Add 1mmol of 5-amino-1H-tetrazolium, 1mmol of 7-fluoroisatin, 1mmol of 5,5-dimethyl-1,3-cyclohexanedione, 4mL of water and 98% concentrated sulfuric acid in a 25mL single-necked flask. 0.5mmol, heated to 80°C, and reacted for 16h under stirring. The reaction solution was cooled to 40-50°C, poured into 30 mL of cold water, and centrifuged to obtain a solid. The solid was recrystallized with 4 mL of acetonitrile, and the recrystallized solid was vacuum-dried at a vacuum degree of 0.1Mpa and a temperature of 50°C to obtain spiro[indoline-3,9'-tetrazole[5,1-b ]quinazoline]-2,8'(5'hydrogen)-one derivatives. Yield: 83%, white solid; mp297-299℃; 1 H NMR (400MHz, DMSO-d 6 )δ12.14(s,1H),11.47(s,1H),7.51–7.11(m,1H),7.02(d,J=7.1Hz,1H),6.98–6.66(m,1H),2.67(dd ,J=38.8,17.1Hz,2H),2.18(dd,J=41.4,16.3Hz,2H),1.05(d,J=11.1Hz,6H); 13 C NMR (101MHz, DMSO-d 6 )δ193.34, 173.38, 153.46, 148.65, 145.67, 132.63, 130.18, 123.67, 120.25, 117....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com