Curcumin derivative and application of curcumin derivative as cannabinoid receptor modulator
A technology of curcumin derivatives and curcumin, which is applied in the direction of drug combination, active ingredients of heterocyclic compounds, digestive system, etc., can solve the problems of limited application, less absorption, and excessive metabolism, and achieve the effect of reducing deposition and promoting degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Example 1: Synthetic process of tetrahydrocurcumin derivatives
[0021] On the basis of summarizing the synthetic methods of curcumin-related analogues, the following synthetic routes were designed to synthesize the target compounds.
[0022]
[0023] Wherein R1 and R2 are each a pyrrole ring or a similar structure.
example 2
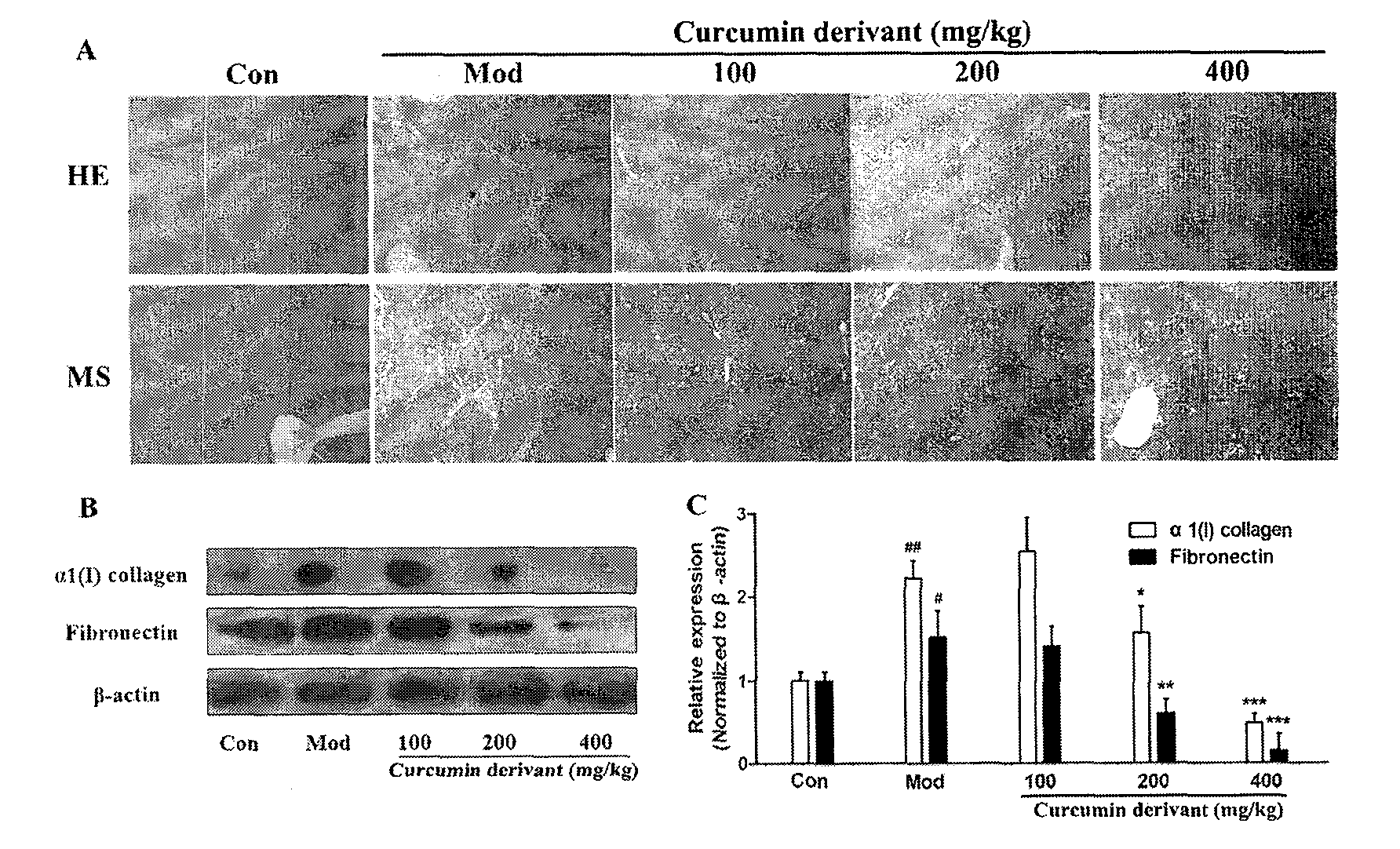
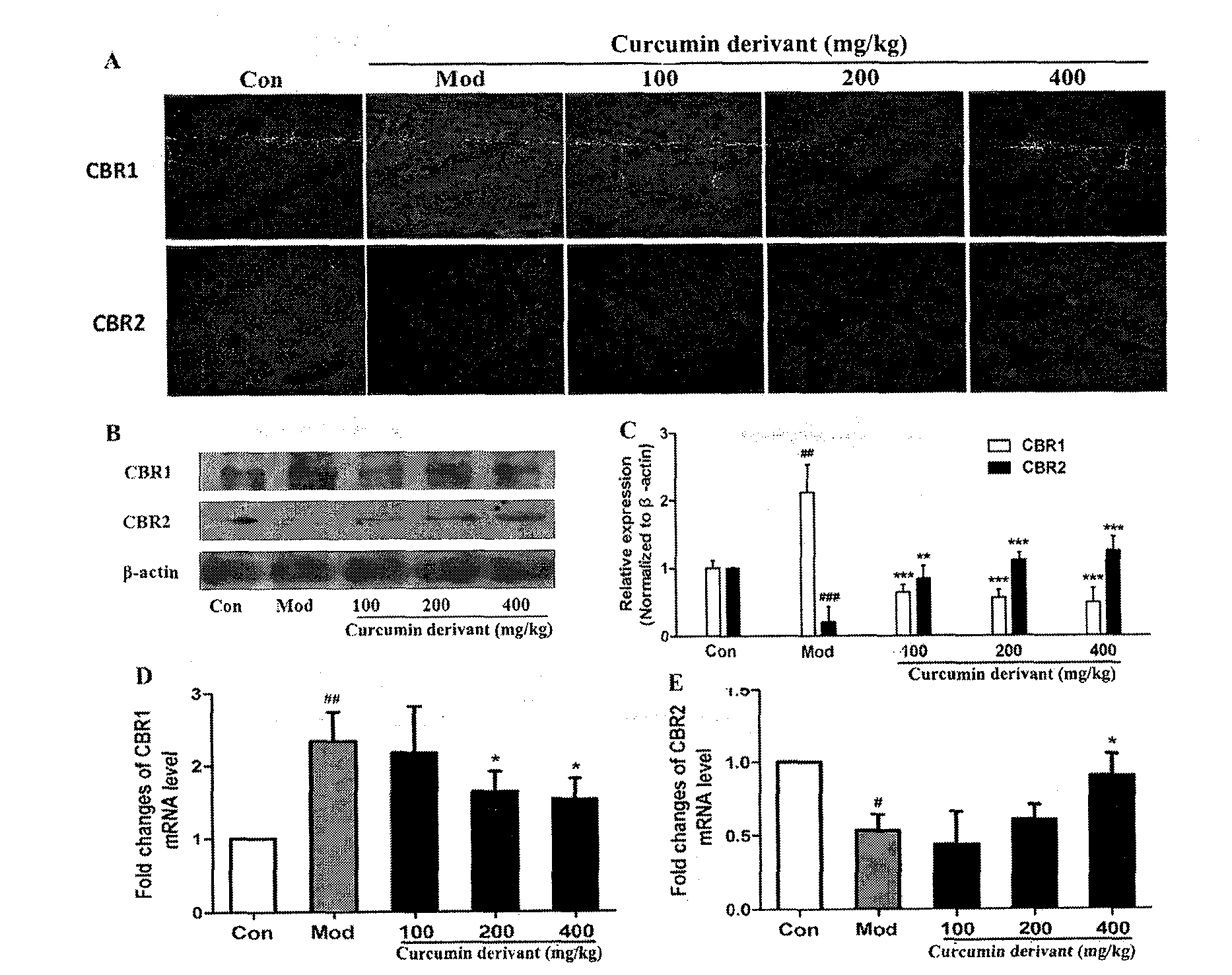
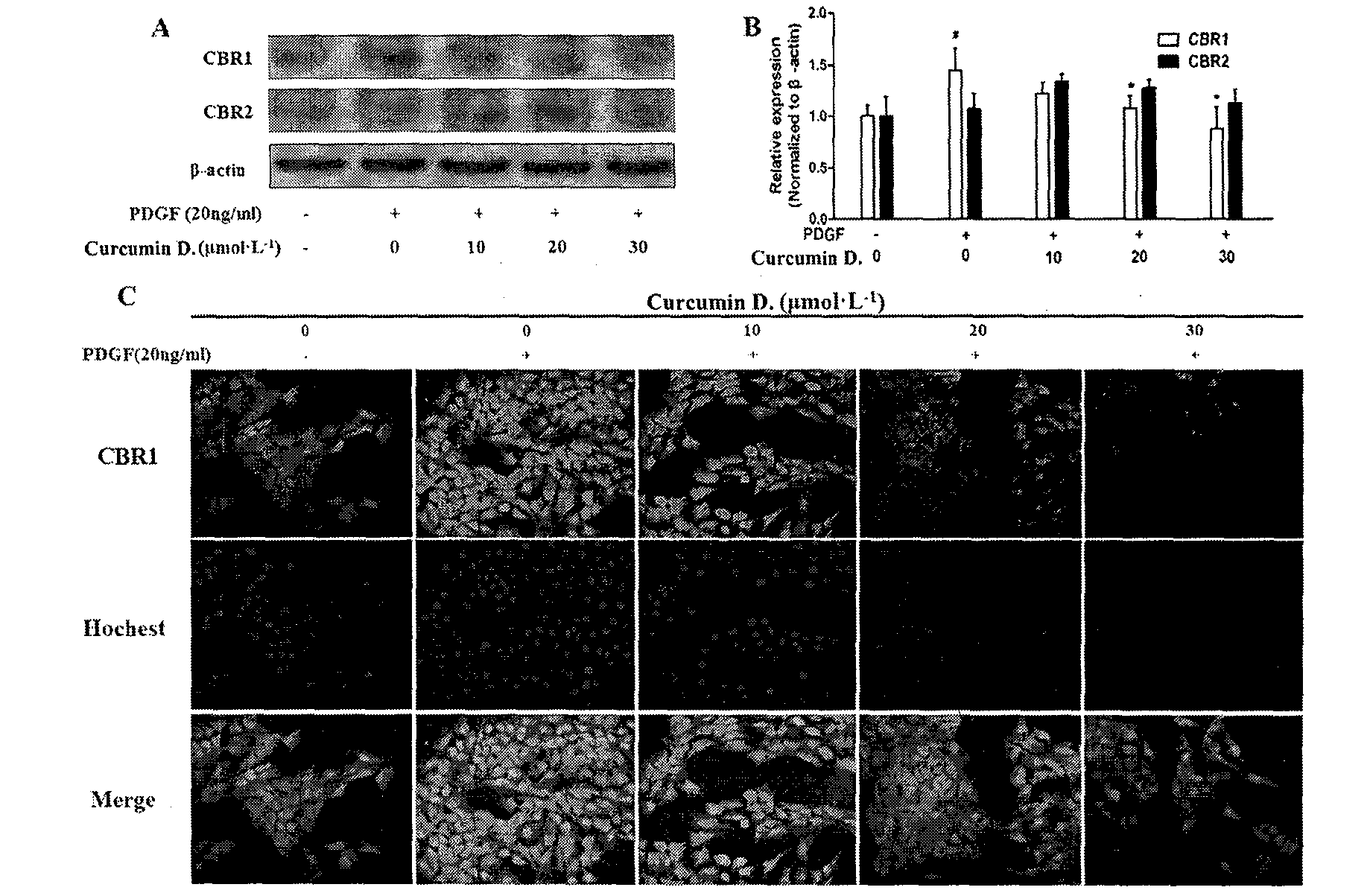
[0024] Example 2: Curcumin derivatives inhibit the occurrence and development of liver fibrosis
Embodiment 1
[0025] Example 1: Curcumin protects liver damage and inhibits CCL 4 Induces hepatic fibrogenesis in rat liver tissue.
[0026] 1. Experimental materials
[0027]1.1 Experimental animals
[0028] Several SPF grade male SD rats (Shanghai Slack Company, SCXK (Shanghai) 2012-0015).
[0029] 1.2 Experimental drugs
[0030] Curcumin derivatives (prepared and synthesized in laboratory, content ≥99.8%)
[0031] 1.3 Experimental reagents
[0032] CCl 4 (domestic analytically pure) mixed with olive oil (Shandong Luhua Co., Ltd.) 1:1 to make 50% CCl 4 Organic solvent for modeling; BCA kit (PIERCE); primary antibody collagen and Fibronectin (Epitomics), β-actin (Sigma); sodium pentobarbital (Shanghai Chemical Reagent Company); absolute ethanol (AR, Nanjing Chemical Reagent Co., Ltd.); formaldehyde (AR, Shanghai Chemical Reagent Company); reagents used in Western Blot experiments: 1.5M Tris-HCl, 1M Tris-HCl, 30% acrylamide solution, TBST, Running buffer, Transfer buffer, 5% skimmed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com