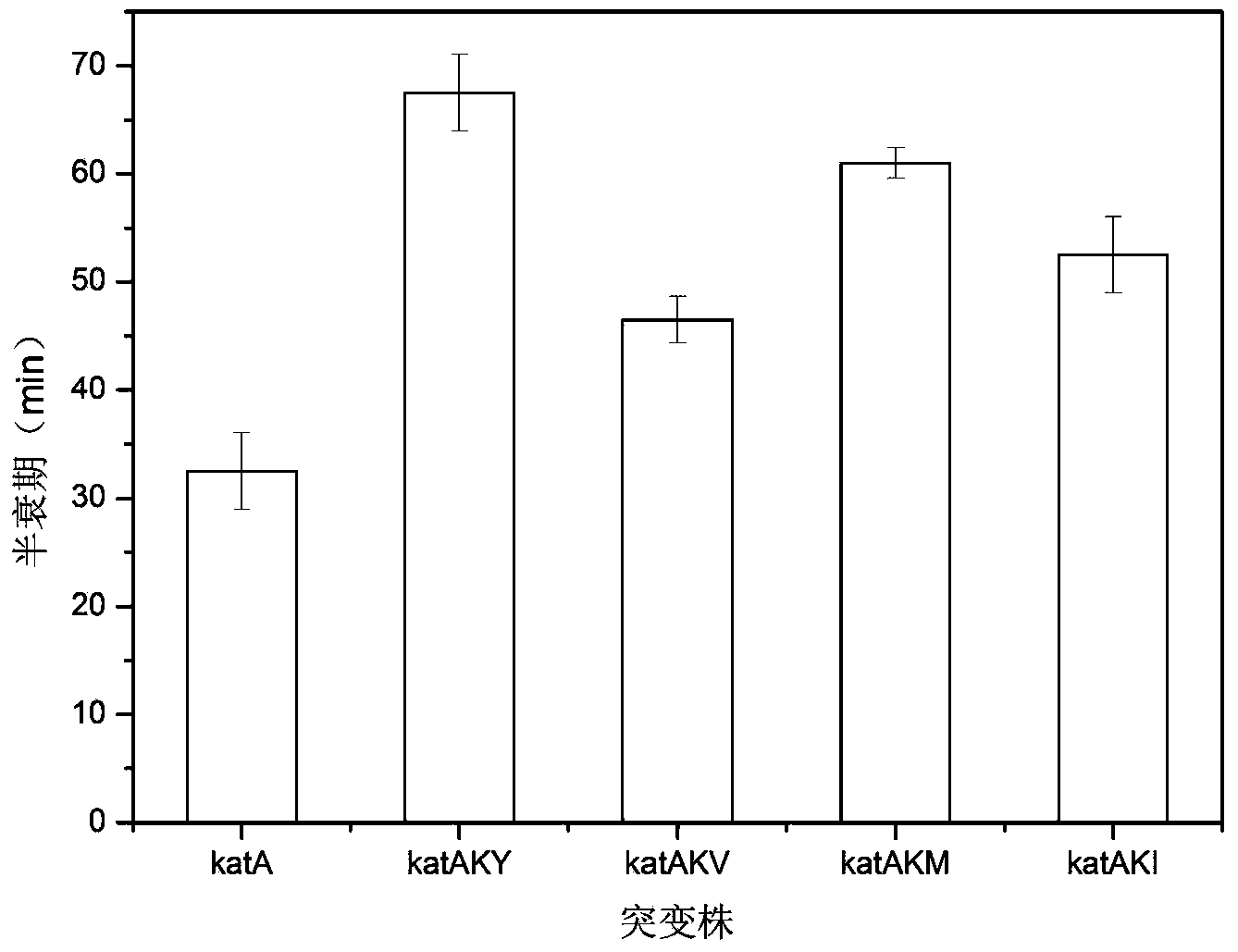Catalase mutant with improved enzyme activity and heat stability
A catalase and thermal stability technology, applied in the field of bioengineering, can solve problems such as temperature sensitivity, and achieve the effect of improving yield and enzymatic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The construction of embodiment 1 mutant expression plasmid and the acquisition of recombinant Bacillus subtilis
[0029] 1. Construction of mutant expression vector
[0030] Using Bacillus subtilis pSTOP1622-katA plasmid (a recombinant Bacillus subtilis with high catalase production and its construction method and application, application number: 201110328753.1; Plasmid system for the intracellular production and purification of affinity-tagged proteins in Bacillus megaterium, Biedendieck R, Yang Y, Deckwer WD, Malten M, Jahn D) were used as templates to amplify plasmids containing mutant genes in vitro by PCR.
[0031] The primers used for site-directed mutagenesis were:
[0032] katAKY primer1: 5'-ACCCGCGCGGATTTGCTGTTTTATTTTTATACTGAAGAAGGAAA-3'
[0033] katAKY primer2: 5'-TTTCCTTCTTCAGTATAAAAATAAACAGCAAATCCGCGCGGGT-3'
[0034] katAKV primer1: 5'-CCCGCGCGGATTTGCTGTTGTATTTTATACTGAAGAAGG-3'
[0035] katAKV primer2: 5'-CCTTCTTCAGTATAAAATACAACAGCAAATCCGCGCGGG-3'
[0036...
Embodiment 2
[0049] Expression of embodiment 2 mutant CAT
[0050] Activation medium: 6% (w / w) wort, pH 7.0-7.5.
[0051] Fermentation medium (g / L): Glucose 10, NaNO 3 5. MgSO 4 ·7H 2 O0.5, Na 2 HPO 4 9.52, KH 2 PO 4 0.6, FeSO 4 ·7H 2 O0.0025, pH natural; xylose induction concentration was 0.5% (w / w).
[0052] Pick the CAT-producing recombinant Bacillus subtilis screened from the LB plate, and inoculate 1-2 loops under sterile conditions in 25mL activation medium with tetracycline at a final concentration of 20ug / mL, at 37°C. Under 200rpm shaker for 16h, the seed solution was obtained; 5% of the inoculum was transferred into a 250mL Erlenmeyer flask containing 25mL of fermentation medium. The fermentation conditions were 37° C., 200 rpm. After 56 hours of fermentation, samples were taken every 4 hours, and the original strain of Bacillus subtilis (B.subtilis WSHDZ-01 / pSTOP1622-katA) was used as a control.
Embodiment 3
[0053] Enzymatic properties of CAT before and after embodiment 3 mutation
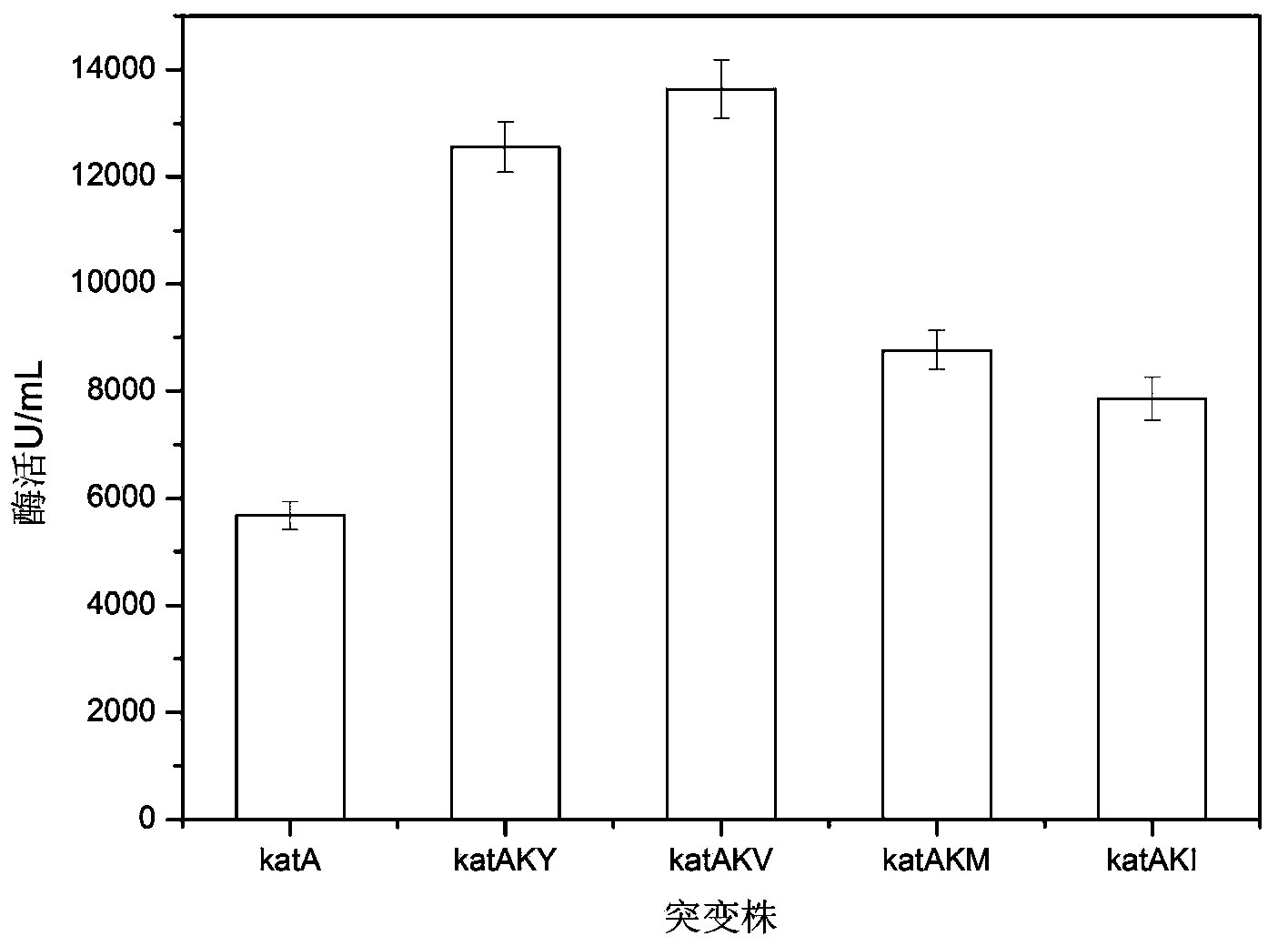
[0054] According to the method described in Example 2, the original Bacillus subtilis (B.subtilis WSHDZ-01 / pSTOP1622-katA, a recombinant Bacillus subtilis with high catalase production and its construction method and application, application number: 201110328753.1) and other mutant strains were fermented, and the extracellular enzyme activity was measured, and the results were as attached figure 1 shown, by attaching figure 1 It can be seen that the enzyme activity of the strain after the mutation is significantly improved compared with that before the mutation, and the highest enzyme activity of katAKV is 2.41 times that before the mutation. The expression levels of the catalase mutants were 2.23, 2.41, 1.46, and 1.38 times that of those before the mutation, respectively.
[0055] Through detection, it is found that the enzyme before mutation is relatively stable at 30°C-50°C, but it is relatively e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com