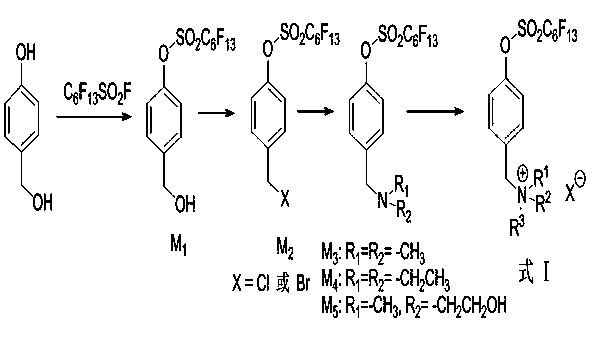Perfluorohexyl sulfonyloxy benzyl cation surfactant as well as preparation method and application thereof
A technology of sulfonyloxybenzyl cation and surfactant, which is applied in the field of perfluorohexylsulfonyloxybenzyl cationic surfactant and its preparation, can solve the problem of great environmental hazards and unsatisfactory fire extinguishing effect, etc. problem, to achieve the effect of excellent fire extinguishing performance, fast water film spreading speed and excellent surface activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 intermediate (M 1 ) preparation
[0032] Add 12.4g (0.1mol) p-hydroxybenzyl alcohol, 17.9g (0.13mol) potassium carbonate, 200mL acetonitrile into a dry 250mL flask, heat to reflux, slowly add 65.3g (0.13mol) perfluorohexylsulfonyl fluoride dropwise, continue The reaction was carried out for 0.5 h, and the end point of the reaction was monitored by TLC. Add 150mL ethyl acetate to the reaction solution, wash 3 times with saturated sodium chloride solution, dry the organic layer with anhydrous sodium sulfate, and remove the solvent to obtain the colorless liquid intermediate perfluorohexylsulfonic acid-4-(hydroxymethyl ) phenyl ester (M 1 ), 44.53g, and the yield was 84%. 1 H NMR (400MHz, CDCl 3 ):δ2.50(s,1H,-OH),4.61(s,2H,-CH 2 -),6.89(d,2H,J=7.8Hz,phH),7.33(d,2H,J=7.8Hz,phH); 19 F NMR (376MHz, CDCl 3 ):δ-126.494(2F),-123.057(2F),-122.182(2F),-120.690(2F),-112.682(2F),-81.136(3F);MS(EI):506.03(M + ) (calculated value: 505.99).
Embodiment 2
[0033] Embodiment 2 intermediate (M 2 ) preparation
[0034] Add 55.6g (0.11mol) perfluorohexylsulfonate-4-(hydroxymethyl)phenyl ester (M 1 ), 200mL of 1,4-dioxane, slowly add 19.47g (0.165mol) of thionyl chloride dropwise under an ice bath, react for 30min after the drop is complete, raise the temperature to 60°C and continue the reaction for 1.0h, and monitor the end of the reaction by TLC. Slowly poured into ice water, added 200mL ethyl acetate, washed three times with saturated sodium chloride solution, dried the organic layer with anhydrous sodium sulfate, and precipitated to obtain a colorless liquid intermediate perfluorohexylsulfonic acid-4-(chloromethyl base) phenyl ester (M 2 ) 45.0g, the yield is 78%. 1 H NMR (400MHz, CDCl 3 ):δ3.42(s,2H,-CH 2 -),7.22(d,2H,J=3.9Hz,phH),7.41(d,2H,J=4.2Hz,phH); 19 F NMR (376MHz, CDCl 3 ):δ-126.494(2F),-123.057(2F),-122.182(2F),-120.690(2F),-112.682(2F),-81.136(3F); MS(EI):524.02(M + ) (calculated value: 523.95).
Embodiment 3
[0035] Embodiment 3 intermediate (M 3 ~ M 5 ) preparation
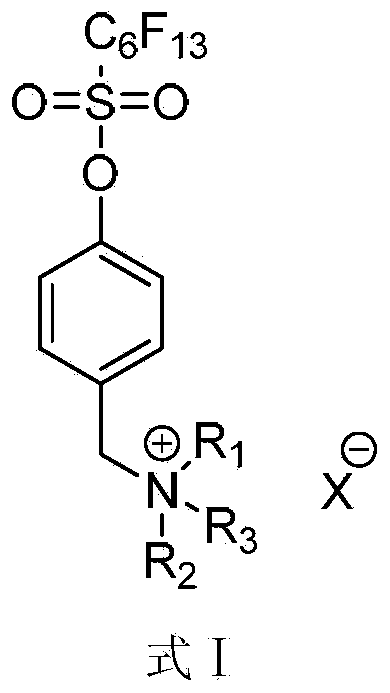
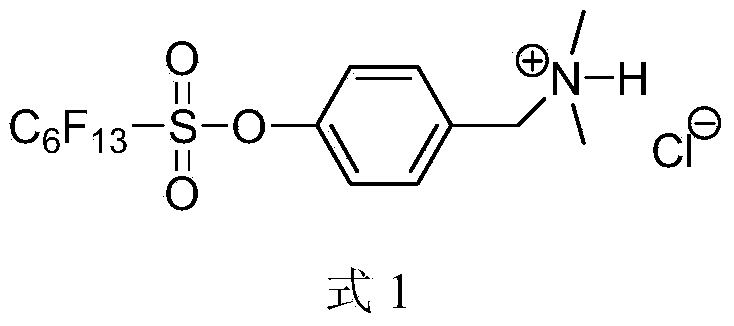
[0036] Add 6.36g (0.015mol) perfluorohexylsulfonate-4-(chloromethyl)phenyl ester (M 2 ), 8.4g (0.06mol) of potassium carbonate, 1.46g (0.018mol) of dimethylamine hydrochloride, 100mL of acetonitrile, reacted at 70-80°C for 2.0h, monitored by TLC, and the reaction was completed. Add 200mL ethyl acetate to the reaction solution, wash with saturated sodium chloride solution three times, dry the organic layer with anhydrous sodium sulfate, and remove the solvent to obtain the colorless liquid intermediate perfluorohexylsulfonic acid-4-(N,N- Dimethylaminomethyl) phenyl ester (M 3 ) 5.85g, the yield is 75%. 1 H NMR (400MHz, CDCl 3 ):δ3.07(s,6H,CH 3 ),4.66(s,2H,CH 2 ),7.69(d,2H,J=8.8Hz,phH),7.79(d,2H,J=8.8Hz,phH); 19 FNMR (376MHz, CDCl 3 ):δ-126.494(2F),-123.057(2F),-122.182(2F),-120.690(2F),-112.682(2F),-81.136(3F); MS(EI):533.04(M + ) (calculated value: 533.03).
[0037] Add 6.36g (0.015mol) perfluorohexylsulfon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com