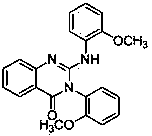
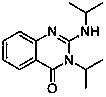
Method for preparing 2-amino-4(3H)-quinazolinones
A technology for aminoquinazoline and ketone compounds, which is applied in the field of catalytic preparation of 2-aminoquinazolin-4-one compounds, and can solve the problem of high reaction cost, high process complexity, complex and rare starting materials, and too many reaction steps and other problems, to achieve the effect of simple and controllable preparation process, simple post-treatment and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
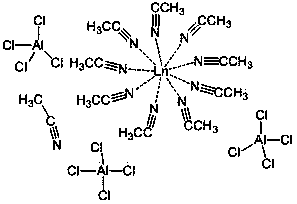
[0037] Example 1: Catalyst [La(CH 3 CN) 9 ] 3+ [(AlCl 4 ) 3 ] 3– ·CH 3 Synthesis of CN
[0038] In the reaction flask that has been dehydrated and deoxygenated, the molar ratio is 1:3 under the protection of argon, and weigh 0.61 g (2.5 mmol) of LaCl 3 And 1.00 g (7.5 mmol) anhydrous AlCl 3 , Add 25 mL of acetonitrile to dissolve, stir at room temperature for 24 hours, centrifuge, take the supernatant, concentrate and stand in a refrigerator at 0 ℃, the obtained crystal is [La(CH 3 CN) 9 ] 3+ [(AlCl 4 ) 3 ] 3– ·CH 3 CN, the yield is 40%.
[0039] The product is white, elemental analysis: La, (13.16±0.5)%, Cl, (40.29±0.5)%.
Embodiment 2
[0040] Example 2: Catalyst [Nd(CH 3 CN) 9 ] 3+ [(AlCl 4 ) 3 ] 3– ·CH 3 Synthesis of CN
[0041] Weigh 0.63 g (2.5 mmol) of NdCl in a reaction flask that has been dehydrated and deoxygenated under the protection of argon gas at a molar ratio of 1:3 3 And 1.00 g (7.5 mmol) anhydrous AlCl 3 , Add 25 mL of acetonitrile to dissolve, stir at room temperature for 24 hours, centrifuge, take the supernatant, concentrate and stand in the refrigerator at 0 ℃, the obtained crystal is [Nd (CH 3 CN) 9 ] 3+ [(AlCl 4 ) 3 ] 3– ·CH 3 CN, the yield is 45%.
[0042] The product is lavender. Elemental analysis: Nd, (13.59±0.5)%, Cl, (40.09±0.5)%.
Embodiment 3
[0043] Example 3: Catalyst [Sm(CH 3 CN) 9 ] 3+ [(AlCl 4 ) 3 ] 3– ·CH 3 Synthesis of CN
[0044] Weigh out 0.64 g (2.5 mmol) of SmCl in a reaction flask that has been dehydrated and deoxygenated under the protection of argon gas at a molar ratio of 1:3 3 And 1.00 g (7.5 mmol) anhydrous AlCl 3 , Add 25 mL of acetonitrile to dissolve, stir at room temperature for 24 hours, centrifuge, take the supernatant, concentrate and stand in a refrigerator at 0 ℃, the obtained crystal is [Sm (CH 3 CN) 9 ] 3+ [(AlCl 4 ) 3 ] 3– ·CH 3 CN, the yield was 53%.
[0045] The product is light yellow. Elemental analysis: Sm, (14.10±0.5)%, Cl, (39.90±0.5)%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com