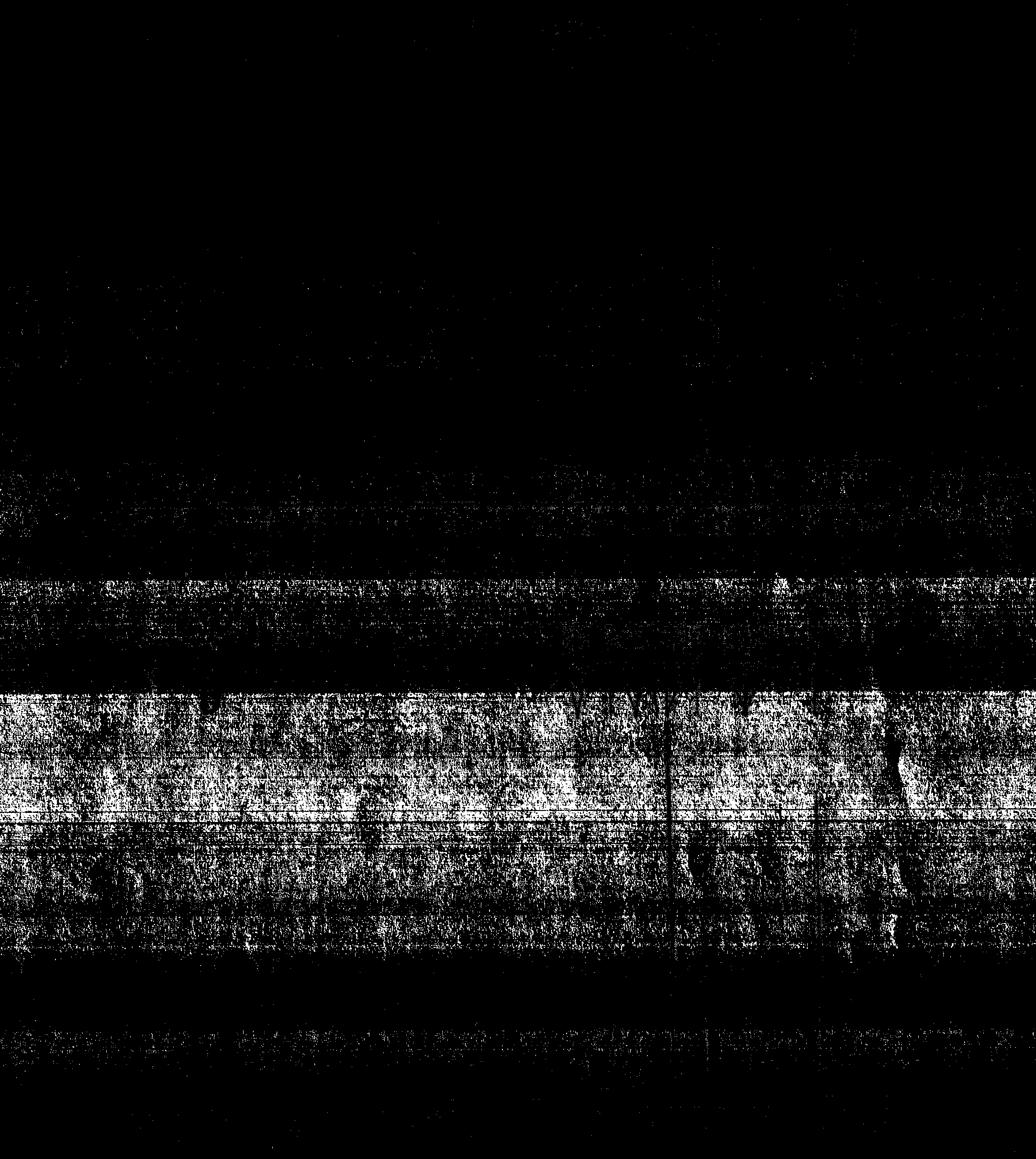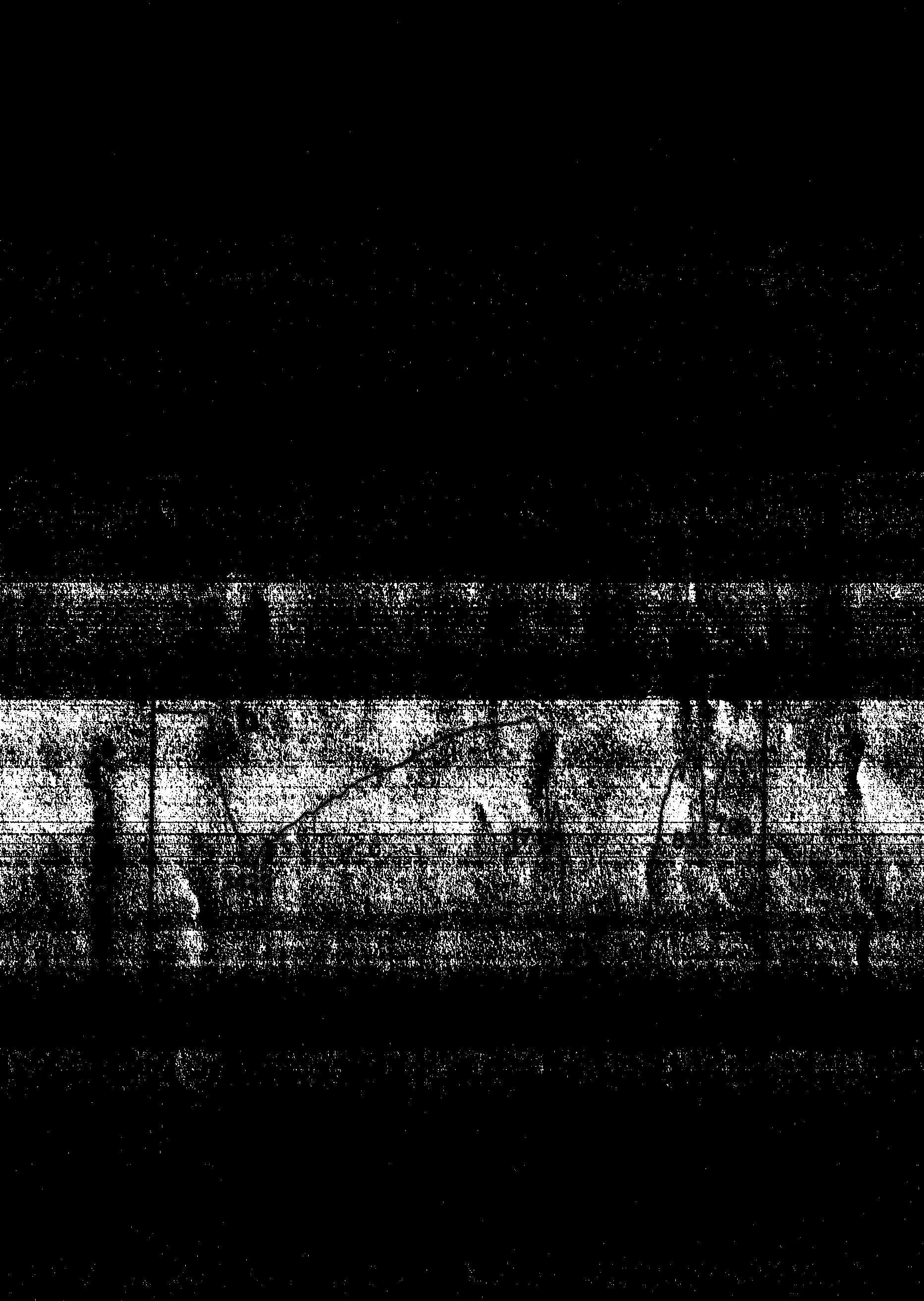Preparation method of aminoanthraquinone polymer
A technology of aminoanthraquinone and polymer, which is applied in the field of new quinone polymers and its preparation, can solve the problems of limited raw material reserves, heavy metal elements polluting the environment, and high energy consumption, etc., and achieves simple and easy preparation process and rich structure types Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] Dissolve ADDAQ (500mg, 1.95mmol) in 40ml of ethyl acetate, add 3 equivalents of anhydrous ferric chloride (1.03g), stir at room temperature for 6h, point samples for analysis of no raw material points, add a large amount of distilled water, filter, and wash with alcohol A tan product was obtained in about 80% yield.
example 2
[0020] At room temperature (25°C), dissolve ADDAQ (500mg, 1.95mmol) in 50ml of acetic acid, cool down to 0°C, and slowly add (NH 4 ) 2 S 2 o 8 (1.2g, 5.26mmol) of saturated aqueous solution 5ml, stirred in an ice bath for 4h, sampled and analyzed without raw material points, then added a large amount of distilled water, stirred and left standing overnight, filtered and washed to obtain a reddish-brown powdery product, the yield was about 85% %.
example 3
[0022] The concentrated sulfuric acid of 2.17ml 98% is slowly added dropwise in DMF, configures 40ml DMF sulfuric acid solution, sulfuric acid concentration is 1mol / L, the ADDAQ of 2mmol is added in 30ml DMF sulfuric acid solution, then its ultrasonic dispersion 15min, impels single 200mg (2mmol) of chromium trioxide was dissolved in 10ml of DMF sulfuric acid solution, and the oxidant was added dropwise to the continuously stirring monomer solution, and the reaction was carried out at room temperature (20°C) for 24h. The reacted solution was suction filtered, and then the product was washed with DMF to obtain a brown product with a yield of about 78%.
[0023] According to the nuclear magnetic spectrum ( figure 1 ), ADDAQ 1 H-NMR shows that δ=14.104 and 13.640 correspond to the two hydrogen atoms on the hydroxyl group respectively, δ=6.955 corresponds to the two hydrogen atoms on the amino group, and δ=7.545~7.366 correspond to the 6, 7 and 8 carbons on the ring respectively ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com